
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Bay 1021189
2. Verquvo
1. 1350653-20-1
2. Verquvo
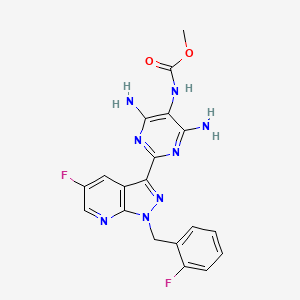
3. Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate
4. Mk-1242
5. Vericiguat [inn]
6. Bay-1021189
7. Bay 1021189
8. Vericiguat [usan]
9. Bay1021189
10. Lv66adm269
11. Methyl N-[4,6-diamino-2-[5-fluoro-1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]carbamate
12. Methyl (4,6-diamino-2-(5-fluoro-1-((2-fluorophenyl)methyl)-1h-pyrazolo(3,4-b)pyridin-3-yl(pyrimidin-5-yl)carbamate
13. Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-5-yl)carbamate
14. Methyl N-(4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-5-yl)carbamate
15. Unii-lv66adm269
16. Methyl {4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}carbamate
17. Vericiguatum
18. Vericiguat [jan]
19. Vericiguat [usan:inn]
20. Vericiguat [who-dd]
21. Bay1021189; Verquvo
22. Schembl429958
23. Vericiguat (jan/usan/inn)
24. Chembl4066936
25. Vericiguat [orange Book]
26. Gtpl10010
27. Chebi:142432
28. Dtxsid001318361
29. Bcp18886
30. Ex-a4694
31. Who 9805
32. Mfcd28502029
33. S9693
34. Zinc72318626
35. Cs-6981
36. Db15456
37. Sb16806
38. Ac-36737
39. Hy-16774
40. Bay1021189bay1021189
41. J3.590.750e
42. D11051
43. P14957
44. A887763
45. Q27283201
46. Bay1021189; Bay 1021189; Bay-1021189; Bay10-21189; Bay-10-21189; Bay 10-21189
47. Methyl 4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-ylcarbamate
48. Methyl{4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}carbamate
| Molecular Weight | 426.4 g/mol |
|---|---|
| Molecular Formula | C19H16F2N8O2 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 5 |
| Exact Mass | 426.13642811 g/mol |
| Monoisotopic Mass | 426.13642811 g/mol |
| Topological Polar Surface Area | 147 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 622 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vericiguat is indicated in adults with symptomatic, chronic heart failure and an ejection fraction of <45% to reduce the risk of cardiovascular death and heart failure-related hospitalization following a hospitalization for heart failure or need for outpatient intravenous diuretics.
Treatment of symptomatic chronic heart failure
By directly stimulating the increased production of intracellular cyclic guanosine monophosphate (cGMP), vericiguat causes the relaxation of vascular smooth muscle and vasodilation. Vericiguat has a relatively long half-life (~30h) that allows for once-daily dosing. Animal reproduction studies have demonstrated the potential for embryo-fetal toxicity when vericiguat is administered to pregnant females - defects in major vessel and heart formation, as well as spontaneous abortions/resorptions, were observed when vericiguat was administered to pregnant rabbits during organogenesis. The possibility of pregnancy should be excluded prior to beginning therapy with vericiguat, and adequate contraception should be used throughout therapy and for one month following cessation of treatment.
C01
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DX - Other vasodilators used in cardiac diseases
C01DX22 - Vericiguat
Absorption
Following the administration of 10mg of vericiguat by mouth once daily, the average steady-state Cmax and AUC in patients with heart failure is 350 mcg/L and 6,680 mcgh/L, respectively, with a Tmax of 1 hour. The absolute bioavailability of orally-administered vericiguat is approximately 93% when taken with food - co-administration with meals has been shown to reduce pharmacokinetic variability, increase Tmax to roughly 4 hours, and increase Cmax and AUC by 41% and 44%, respectively.
Route of Elimination
Following the oral administration of radiolabeled vericiguat, approximately 53% of the administered radioactivity was recovered in the urine and 45% in the feces. A human mass balance study found that the portion recovered in the urine comprised approximately 40.8% N-glucuronide metabolite, 7.7% other metabolites, and 9% unchanged parent drug, while virtually the entire portion recovered in the feces comprised unchanged vericiguat.
Volume of Distribution
In healthy subjects the steady-state volume of distribution of vericiguat is approximately 44 liters.
Clearance
Vericiguat is a low-clearance drug, with an observed plasma clearance of 1.6 L/h in healthy volunteers and 1.3 L/h in patients with systolic heart failure.
Vericiguat is primarily metabolized via phase II conjugation reactions, with CYP-mediated oxidative metabolism comprising a small (<5%) portion of its overall biotransformation. The major inactive metabolite, vericiguat N-glucuronide (M1), is formed by UGT1A9 and, to a lesser extent, UGT1A1. Other identified metabolites include a denbenzylated compound and an M15 metabolite thought to be the result of oxidative metabolism, although these metabolites are poorly characterized.
In patients with heart failure, the half-life of vericiguat is 30 hours.
Heart failure (HF) involves, amongst other morphologic and physiologic changes, the impaired synthesis of nitric oxide (NO) and decreased activity of soluble guanylate cyclase (sGC). Functioning normally, NO binds to sGC and stimulates the synthesis of intracellular cyclic guanosine monophosphate (cGMP), a second messenger involved in the maintenance of vascular tone, as well as cardiac contractility and remodeling. Defects in this pathway are thought to contribute to the myocardial and vascular dysfunction associated with heart failure and are therefore a desirable target in its treatment. Vericiguat directly stimulates sGC by binding to a target site on its beta-subunit, bypassing the need for NO-mediated activation, and in doing so causes an increase in the production of intracellular cGMP that results in vascular smooth muscle relaxation and vasodilation.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37796
Submission : 2022-12-23
Status : Active
Type : II
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40776
Submission : 2024-11-07
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2024-07-29
Pay. Date : 2024-07-09
DMF Number : 37984
Submission : 2023-02-27
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2025-01-10
Pay. Date : 2024-11-21
DMF Number : 40665
Submission : 2024-11-22
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2024-11-08
Pay. Date : 2024-09-24
DMF Number : 40459
Submission : 2024-09-28
Status : Active
Type : II

NDC Package Code : 72640-030
Start Marketing Date : 2024-09-28
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Bayer granted Dr. Reddy’s non-exclusive rights for Vericiguat under the brand name Gantra in India, indicated for adults with symptomatic chronic heart failure with reduced ejection fraction.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Dr. Reddy's Laboratories
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership May 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Dr. Reddy's Laboratories
Deal Size : Undisclosed
Deal Type : Partnership
Bayer and Dr. Reddy’s Sign Marketing Agreement for Vericiguat™ in India
Details : Bayer granted Dr. Reddy’s non-exclusive rights for Vericiguat under the brand name Gantra in India, indicated for adults with symptomatic chronic heart failure with reduced ejection fraction.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
May 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Verquvo (vericiguat) is an oral once daily stimulator of soluble guanylate cyclase (sGC), an important enzyme in the NO-sGC-cGMP signaling pathway and currently in pediatric patients aged > 28 days to 18 years with heart failure.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Merck & Co
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Merck & Co
Deal Size : Inapplicable
Deal Type : Inapplicable
Bayer Starts Phase II/III Study with Vericiguat in Children with Heart Failure
Details : Verquvo (vericiguat) is an oral once daily stimulator of soluble guanylate cyclase (sGC), an important enzyme in the NO-sGC-cGMP signaling pathway and currently in pediatric patients aged > 28 days to 18 years with heart failure.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Verquvo (Vericiguat) 2.5 mg, 5 mg, and 10 mg is an oral once daily stimulator of soluble guanylate cyclase (sGC), an important enzyme in the nitric oxide (NO) signaling pathway.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Verquvo™ (vericiguat) Approved in China to Treat Patients with Chronic Heart Failure and Reduced...
Details : Verquvo (Vericiguat) 2.5 mg, 5 mg, and 10 mg is an oral once daily stimulator of soluble guanylate cyclase (sGC), an important enzyme in the nitric oxide (NO) signaling pathway.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
A pivotal Phase 3 (VICTOR) study has been initiated for VERQUVO® (vericiguat) in patients with chronic heart failure and reduced ejection fraction of 40% or less who have not had a recent worsening heart failure event.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 11, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Merck Announces Initiation of Phase 3 Study Evaluating VERQUVO® (Vericiguat) in Patients with Chr...
Details : A pivotal Phase 3 (VICTOR) study has been initiated for VERQUVO® (vericiguat) in patients with chronic heart failure and reduced ejection fraction of 40% or less who have not had a recent worsening heart failure event.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
November 11, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
VERQUVO is a stimulator of soluble guanylate cyclase (sGC), an important enzyme in the nitric oxide (NO) signaling pathway.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
VERQUVO® (Vericiguat) Approved in the European Union
Details : VERQUVO is a stimulator of soluble guanylate cyclase (sGC), an important enzyme in the nitric oxide (NO) signaling pathway.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vericiguat will be the first treatment option to have been studied specifically in patients after a recent decompensation in order to help break the cycle of worsening events, reduce the risk of re-hospitalization.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Positive CHMP Opinion for Bayer’s New Symptomatic Chronic Heart Failure Treatment Vericiguat
Details : Vericiguat will be the first treatment option to have been studied specifically in patients after a recent decompensation in order to help break the cycle of worsening events, reduce the risk of re-hospitalization.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
VERQUVO is approved for reduction of risk of cardiovascular death and heart failure (hf) hospitalization following a hospitalization for HF or need for outpatient intravenous (IV) diuretics in adults with symptomatic chronic heart failure and ejection fraction less than 45%.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Bayer AG
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 20, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Bayer AG
Deal Size : Inapplicable
Deal Type : Inapplicable
Merck Announces U.S. FDA Approval of VERQUVO® (Vericiguat)
Details : VERQUVO is approved for reduction of risk of cardiovascular death and heart failure (hf) hospitalization following a hospitalization for HF or need for outpatient intravenous (IV) diuretics in adults with symptomatic chronic heart failure and ejection fr...
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 20, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vericiguat is an investigational oral, once-daily, first-in-class soluble guanylate cyclase (sGC)-stimulator being developed to treat patients with symptomatic chronic heart failure with an ejection fraction less than 45%.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 28, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Vericiguat is an investigational oral, once-daily, first-in-class soluble guanylate cyclase (sGC)-stimulator being developed to treat patients with symptomatic chronic heart failure with an ejection fraction less than 45%.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
August 28, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The application is based on results from the Phase 3 VICTORIA trial, which is the first contemporary outcomes study focused solely on a population with worsening chronic heart failure at high risk for cardiovascular mortality and repeated heart failure hospitalizations.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Bayer AG
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 16, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Bayer AG
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Grants Priority Review to Merck’s New Drug Application for Vericiguat
Details : The application is based on results from the Phase 3 VICTORIA trial, which is the first contemporary outcomes study focused solely on a population with worsening chronic heart failure at high risk for cardiovascular mortality and repeated heart failure h...
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 16, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Regulatory submissions based on positive data from Phase III VICTORIA study recently published in the New England Journal of Medicine.
Lead Product(s): Vericiguat
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Verquvo
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 06, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vericiguat
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Regulatory submissions based on positive data from Phase III VICTORIA study recently published in the New England Journal of Medicine.
Product Name : Verquvo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 06, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]5-Fluoro-1-(2-Fluorobenzyl)-1H-Pyrazolo[3,4-B]Pyri...
CAS Number : 1350653-26-7
End Use API : Vericiguat
About The Company : Established in May 2012, Shandong Loncom Pharmaceutical operates as a fully owned subsidiary of Shandong Bestcomm Pharmaceutical Co., Ltd. Situated in the Qihe ...
2-AMINO-2, 6-DIHYROXY PYRIMIDINE
CAS Number : 873-83-6
End Use API : Vericiguat
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

CAS Number : 107-91-5
End Use API : Vericiguat
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

5-fluoro-1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]...
CAS Number : 1350653-26-7
End Use API : Vericiguat
About The Company : Saptagir Laboratories Private incorporated in 2016, is a manufacturer and supplier of Active Pharmaceutical Ingredients (APIs) and Intermediates for a wide rang...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
14
PharmaCompass offers a list of Vericiguat API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vericiguat manufacturer or Vericiguat supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vericiguat manufacturer or Vericiguat supplier.
PharmaCompass also assists you with knowing the Vericiguat API Price utilized in the formulation of products. Vericiguat API Price is not always fixed or binding as the Vericiguat Price is obtained through a variety of data sources. The Vericiguat Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vericiguat manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vericiguat, including repackagers and relabelers. The FDA regulates Vericiguat manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vericiguat API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vericiguat manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vericiguat supplier is an individual or a company that provides Vericiguat active pharmaceutical ingredient (API) or Vericiguat finished formulations upon request. The Vericiguat suppliers may include Vericiguat API manufacturers, exporters, distributors and traders.
click here to find a list of Vericiguat suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Vericiguat DMF (Drug Master File) is a document detailing the whole manufacturing process of Vericiguat active pharmaceutical ingredient (API) in detail. Different forms of Vericiguat DMFs exist exist since differing nations have different regulations, such as Vericiguat USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Vericiguat DMF submitted to regulatory agencies in the US is known as a USDMF. Vericiguat USDMF includes data on Vericiguat's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Vericiguat USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Vericiguat suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Vericiguat as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Vericiguat API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Vericiguat as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Vericiguat and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Vericiguat NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Vericiguat suppliers with NDC on PharmaCompass.
Vericiguat Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vericiguat GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vericiguat GMP manufacturer or Vericiguat GMP API supplier for your needs.
A Vericiguat CoA (Certificate of Analysis) is a formal document that attests to Vericiguat's compliance with Vericiguat specifications and serves as a tool for batch-level quality control.
Vericiguat CoA mostly includes findings from lab analyses of a specific batch. For each Vericiguat CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vericiguat may be tested according to a variety of international standards, such as European Pharmacopoeia (Vericiguat EP), Vericiguat JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vericiguat USP).
