
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF
0
FDA Orange Book

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
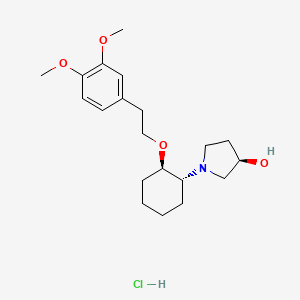

1. Rsd 1235
2. Rsd-1235
3. Rsd1235
4. Vernakalant
1. 748810-28-8
2. Vernakalant Hcl
3. Brinavess
4. Kynapid
5. Vernakalant (hydrochloride)
6. Rsd1235
7. Vernakalant Hydrochloride [usan]
8. Rsd-1235
9. 7g4j1zd9uq
10. (3r)-1-((1r,2r)-2-(2-(3,4-dimethoxyphenyl)ethoxy)cyclohexyl)pyrrolidin-3-ol Hydrochloride
11. Vernakalant Hydrochloride (usan)
12. Rsd 1235
13. Unii-7g4j1zd9uq
14. (3r)-1-[(1r,2r)-2-[2-(3,4-dimethoxyphenyl)ethoxy]cyclohexyl]pyrrolidin-3-ol Hydrochloride
15. Schembl439679
16. Chembl2107383
17. Dtxsid30976007
18. (3r)-1-[(1r,2r)-2-[2-(3,4-dimethoxyphenyl)ethoxy]cyclohexyl]pyrrolidin-3-ol;hydrochloride
19. Yeb81028
20. Vernakalant Hydrochloride [mi]
21. Cs-0799
22. Mk-6621
23. Vernakalant Hydrochloride [mart.]
24. As-58436
25. Hy-14183
26. Vernakalant Hydrochloride [who-dd]
27. Vernakalant Hydrochloride [ema Epar]
28. C76396
29. D06665
30. A915186
31. Q27268233
32. Rsd1235 Hydrochloride;rsd 1235 Hydrochloride;rsd-1235 Hydrochloride
33. (3r)-1-[(1r,2r)-2-[2-(3,4-dimethoxyphenyl)ethoxy]cyclohexyl]-3-pyrrolidinol Hydrochloride
34. 1-{2-[2-(3,4-dimethoxyphenyl)ethoxy]cyclohexyl}pyrrolidin-3-ol--hydrogen Chloride (1/1)
35. (3r)-1-[(1r,2r)-2-[2-(3,4-dimethoxyphenyl)ethoxy]cyclohexyl]-3-pyrrolidinol Monohydrochloride
36. 3-pyrrolidinol, 1-((1r,2r)-2-(2-(3,4-dimethoxyphenyl)ethoxy)cyclohexyl)-, Hydrochloride, (3r)-
37. 605683-48-5
| Molecular Weight | 385.9 g/mol |
|---|---|
| Molecular Formula | C20H32ClNO4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 385.2019862 g/mol |
| Monoisotopic Mass | 385.2019862 g/mol |
| Topological Polar Surface Area | 51.2 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 394 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Rapid conversion of recent onset atrial fibrillation to sinus rhythm in adults:
- for non-surgery patients: atrial fibrillation < /= 7 days duration;
- for post-cardiac surgery patients: atrial fibrillation < /= 3 days duration.
C01BG11
Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?