
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
Listed Suppliers
0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
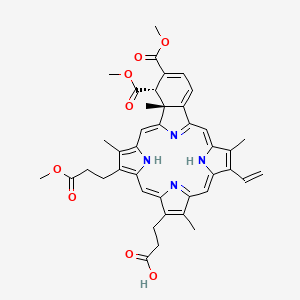

1. 18-ethenyl-4,4a-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-23h,25h-benzo(b)porphine-9,13-dipropanoic Acid Monomethyl Ester
2. Benzoporphyrin Derivative Monoacid Ring A
3. Bpd Verteporfin
4. Bpd-ma
5. Verteporfin
6. Verteporphin
1. Bpd Verteporfin
2. Bpd-ma(sub C)
3. Verteporfin C Isomer
4. Wu713d62n9
5. Verteporphin
6. Cl-315555
7. 133513-12-9
8. (1): 3-[(23s,24r)-14-ethenyl-5-(3-methoxy-3-oxopropyl)-22,23-bis(methoxycarbonyl)-4,10,15,24-tetramethyl-25,26,27,28-tetraazahexacyclo[16.6.1.13,6.18,11.113,16.019,24]octacosa-1,3,5,7,9,11(27),12,14,16,18(25),19,21-dodecaen-9-yl]propanoic Acid.
9. (2r,2(1)s)-8-ethenyl-2(1),2(2)-bis(methoxycarbonyl)-17-(3-methoxy-3-oxopropyl)-2,7,12,18-tetramethyl-2,2(1)-dihydrobenzo[b]porphyrin-13-propanoic Acid
10. (4s,4ar)-18-ethenyl-4,4a-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-23h,25h-benzo[b]porphine-9,13-dipropanoic Acid 9-methyl Ester
11. 23h,25h-benzo(b)porphine-9,13-dipropanoic Acid, 18-ethenyl-4,4a-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-, 9-monomethyl Ester, Trans-
12. 3-[(1z,6z,12z,17z,23s,24r)-14-ethenyl-22,23-bis(methoxycarbonyl)-5-(3-methoxy-3-oxopropyl)-4,10,15,24-tetramethyl-25,26,27,28-tetraazahexacyclo[16.6.1.1(3,6).1(8,11).113,16.019,24]octacosa-1,3(28),4,6,8,10,12,14,16(26),17,19,21-dodecaen-9-yl]propanoic Acid
13. Bpd-ma-a1
14. Unii-0x9pa28k43
15. Verteporfin [usan:usp:inn:ban]
16. Verteporfin-mac
17. Verteporfin C5 Isomer
18. Schembl6219
19. (+-)-trans-3,4-dicarboxy-4,4a-dihydro-4a,8,14,19-tetramethyl-18-vinyl-23h,25h-benzo(b)porphine-9,13-dipropionic Acid, 3,4,9-trimethyl Ester Mixture With (+-)-trans-3,4-dicarboxy-4,4a-dihydro-4a,8,14,19-tetramethyl-18-vinyl-23h,25h-benzo(b)porphine-9,13-dipropionic Acid, 3,4,13-trimethyl Ester
20. Trans-3,4-dicarboxy-4,4a-dihydro-4a,8,14,19-tetramethyl-18-vinyl-23h,25h-benzo(b)porphine-9,13-dipropionic Acid 3,4,9-trimethyl Ester
21. Unii-wu713d62n9
22. Chembl2052016
23. Chembl3822934
24. Schembl14257223
25. Chebi:60775
26. Dtxsid30892511
27. 0x9pa28k43
28. Dtxsid001031353
29. Ex-a1344
30. Nsc836163
31. S1786
32. Ccg-270403
33. Db00460
34. Nsc-836163
35. Ncgc00346712-01
36. Ncgc00346712-02
37. 23h,25h-benzo(b)porphine-9,13-dipropanoic Acid, 18-ethenyl-4,4a-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-, Monomethyl Ester, Trans-
38. Sw219955-1
39. J2.000.745a
40. Ab01566862_01
41. A899099
42. J-005685
43. (2r,21s)-2,21-dihydro-8-vinyl-2,7,12,18-tetramethyl-17-[2-(methoxycarbonyl)ethyl]-21,22-bis(methoxycarbonyl)-21h,23h-benzo[b]porphyrin-13-propionic Acid
44. (2r,21s)-8-ethenyl-2,21-dihydro-13-(3-hydroxy-3-oxopropyl)-17-(3-methoxy-3-oxopropyl)-2,7,12,18-tetramethyl-22h,24h-benzo[b]porphyrin-21,22-dicarboxylic Acid Dimethyl Ester
45. 3-[(23s,24r)-14-ethenyl-22,23-bis(methoxycarbonyl)-5-(3-methoxy-3-oxopropyl)-4,10,15,24-tetramethyl-25,26,27,28-tetrazahexacyclo[16.6.1.13,6.18,11.113,16.019,24]octacosa-1,3,5,7,9,11(27),12,14,16,18(25),19,21-dodecaen-9-yl]propanoic Acid
46. 9-methyl-trans-18-ethenyl-4,4a-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-23h,25h-benzo(b)porphine-9,13-dipropanoate,dl-
| Molecular Weight | 718.8 g/mol |
|---|---|
| Molecular Formula | C41H42N4O8 |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 718.30026431 g/mol |
| Monoisotopic Mass | 718.30026431 g/mol |
| Topological Polar Surface Area | 174 Ų |
| Heavy Atom Count | 53 |
| Formal Charge | 0 |
| Complexity | 1500 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Visudyne |
| PubMed Health | Verteporfin (Injection) |
| Drug Classes | Photosensitizing Agent |
| Drug Label | Visudyne (verteporfin for injection) is a light activated drug used in photodynamic therapy. The finished drug product is a lyophilized dark green cake. Verteporfin is a 1:1 mixture of two regioisomers (I and II), represented by the following struc... |
| Active Ingredient | Verteporfin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 15mg/vial |
| Market Status | Prescription |
| Company | Valeant Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Visudyne |
| PubMed Health | Verteporfin (Injection) |
| Drug Classes | Photosensitizing Agent |
| Drug Label | Visudyne (verteporfin for injection) is a light activated drug used in photodynamic therapy. The finished drug product is a lyophilized dark green cake. Verteporfin is a 1:1 mixture of two regioisomers (I and II), represented by the following struc... |
| Active Ingredient | Verteporfin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 15mg/vial |
| Market Status | Prescription |
| Company | Valeant Pharms |
For the treatment of patients with predominantly classic subfoveal choroidal neovascularization due to age-related macular degeneration, pathologic myopia or presumed ocular histoplasmosis syndrome. Verteporfin can also be used to destroy tumors.
FDA Label
Visudyne is indicated for the treatment of:
- adults with exudative (wet) age-related macular degeneration (AMD) with predominantly classic subfoveal choroidal neovascularisation (CNV) or;
- adults with subfoveal choroidal neovascularisation secondary to pathological myopia.
Verteporfin, otherwise known as benzoporphyrin derivative, is a medication used in conjunction with laser treatment to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular degeneration. Verteporfin accumulates in these abnormal blood vessels and, when stimulated by nonthermal red light with a wavelength of 693 nm in the presence of oxygen, produces highly reactive short-lived singlet oxygen and other reactive oxygen radicals, resulting in local damage to the endothelium and blockage of the vessels.
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
S01LA01
Route of Elimination
Elimination is by the fecal route, with less than 0.01% of the dose recovered in urine.
Metabolized to a small extent to its diacid metabolite by liver and plasma esterases. NADPH-dependent liver enzyme systems (including the cytochrome P450 isozymes) do not appear to play a role in the metabolism of verteporfin.
Following intravenous infusion, verteporfin exhibits a bi-exponential elimination with a terminal elimination half-life of approximately 5-6 hours. Mild hepatic insufficiency increases half-life by approximately 20%.
Verteporfin is transported in the plasma primarily by lipoproteins. Once verteporfin is activated by light in the presence of oxygen, highly reactive, short-lived singlet oxygen and reactive oxygen radicals are generated. Light activation of verteporfin results in local damage to neovascular endothelium, resulting in vessel occlusion. Damaged endothelium is known to release procoagulant and vasoactive factors through the lipo-oxygenase (leukotriene) and cyclo-oxygenase (eicosanoids such as thromboxane) pathways, resulting in platelet aggregation, fibrin clot formation and vasoconstriction. Verteporfin appears to somewhat preferentially accumulate in neovasculature, including choroidal neovasculature. However, animal models indicate that the drug is also present in the retina. As singlet oxygen and reactive oxygen radicals are cytotoxic, Verteporfin can also be used to destroy tumor cells.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
55
PharmaCompass offers a list of Verteporfin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Verteporfin manufacturer or Verteporfin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Verteporfin manufacturer or Verteporfin supplier.
PharmaCompass also assists you with knowing the Verteporfin API Price utilized in the formulation of products. Verteporfin API Price is not always fixed or binding as the Verteporfin Price is obtained through a variety of data sources. The Verteporfin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Verteporfin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Verteporfin, including repackagers and relabelers. The FDA regulates Verteporfin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Verteporfin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Verteporfin supplier is an individual or a company that provides Verteporfin active pharmaceutical ingredient (API) or Verteporfin finished formulations upon request. The Verteporfin suppliers may include Verteporfin API manufacturers, exporters, distributors and traders.
click here to find a list of Verteporfin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Verteporfin DMF (Drug Master File) is a document detailing the whole manufacturing process of Verteporfin active pharmaceutical ingredient (API) in detail. Different forms of Verteporfin DMFs exist exist since differing nations have different regulations, such as Verteporfin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Verteporfin DMF submitted to regulatory agencies in the US is known as a USDMF. Verteporfin USDMF includes data on Verteporfin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Verteporfin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Verteporfin suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Verteporfin Drug Master File in Korea (Verteporfin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Verteporfin. The MFDS reviews the Verteporfin KDMF as part of the drug registration process and uses the information provided in the Verteporfin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Verteporfin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Verteporfin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Verteporfin suppliers with KDMF on PharmaCompass.
Verteporfin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Verteporfin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Verteporfin GMP manufacturer or Verteporfin GMP API supplier for your needs.
A Verteporfin CoA (Certificate of Analysis) is a formal document that attests to Verteporfin's compliance with Verteporfin specifications and serves as a tool for batch-level quality control.
Verteporfin CoA mostly includes findings from lab analyses of a specific batch. For each Verteporfin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Verteporfin may be tested according to a variety of international standards, such as European Pharmacopoeia (Verteporfin EP), Verteporfin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Verteporfin USP).
