
Synopsis
Synopsis
0
JDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
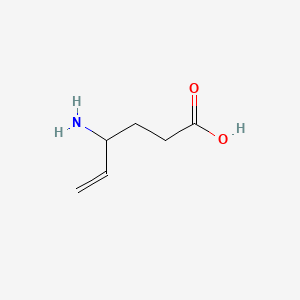

1. Gamma Vinyl Gaba
2. Gamma Vinyl Gamma Aminobutyric Acid
3. Gamma-vinyl-gaba
4. Gamma-vinyl-gamma-aminobutyric Acid
5. Sabril
6. Sabrilex
1. 4-aminohex-5-enoic Acid
2. 60643-86-9
3. Sabril
4. 68506-86-5
5. 4-amino-5-hexenoic Acid
6. Gamma-vinyl Gaba
7. Vigabatrine
8. Gamma-vinyl-gaba
9. 5-hexenoic Acid, 4-amino-
10. Gamma Vinyl Gaba
11. Vigabatrinum
12. Mdl-71754
13. 4-aminohexenoic Acid
14. Gamma-vinyl-gamma-aminobutyric Acid
15. Rmi-71754
16. Cpp-109
17. Gvg
18. Mdl 71,754
19. Cpp109
20. (+/-)-vigabatrin
21. Vinyl Gamma-aminobutyric Acid
22. Gr120krt6k
23. (+/-)-gamma-vinyl Gaba
24. Chembl89598
25. Chebi:63638
26. Vigabatrine [french]
27. Vigabatrinum [latin]
28. (+/-)-4-aminohex-5-enoic Acid
29. Vigabatrina [spanish]
30. Ncgc00016087-06
31. Vigabatrina
32. Sabrilex
33. 4-aminohex-5-enoic Acid/s(+)-gamma-vigabatrin
34. Sabril (tn)
35. Vigabatrin [usan:inn:ban]
36. Rmi-71890
37. Mdl 71754
38. Rmi 71754
39. Hexenoic Acid, 4-amino
40. Sr-01000075653
41. Unii-gr120krt6k
42. (+-)-gamma-vinyl Gaba
43. 4-amino-hex-5-enoic Acid
44. (+/-)-gamma-vinyl-gaba
45. Kigabeq
46. Rac-vigabatrin
47. Mfcd00274076
48. (1)-4-aminohex-5-enoic Acid
49. Cpp 109
50. Vigabatrin Solution
51. Vigabatrin [inn]
52. Prestwick_837
53. Einecs 270-929-6
54. Cas-60643-86-9
55. Mfcd00274577
56. .gamma.-vinyl Gaba
57. .gamma.-vinyl-gaba
58. Spectrum_000368
59. 4-amino-5-hexenoicacid
60. Specplus_000664
61. Vigabatrin [mi]
62. (y)-gamma-vinyl Gaba
63. Vigabatrin [jan]
64. Prestwick0_000501
65. Prestwick1_000501
66. Prestwick2_000501
67. Prestwick3_000501
68. Spectrum3_001825
69. Vigabatrin [usan]
70. (?)-gamma-vinyl Gaba
71. Vigabatrin [vandf]
72. Biomol-nt_000247
73. V 8261
74. Vigabatrin [mart.]
75. Dsstox_cid_21153
76. Dsstox_rid_79637
77. Vigabatrin [usp-rs]
78. Vigabatrin [who-dd]
79. Dsstox_gsid_41153
80. Lopac0_001277
81. M071754
82. Schembl26714
83. Bspbio_000421
84. Bspbio_003469
85. Kbioss_000848
86. Vigabatrin (jan/usp/inn)
87. (a+/-)-gamma-vinyl Gaba
88. Divk1c_006760
89. Spectrum1502036
90. (+/-)-?-vinyl Gaba
91. Spbio_002342
92. Bpbio1_000465
93. Bpbio1_000925
94. Gtpl4821
95. Dtxsid4041153
96. Vigabatrin [orange Book]
97. Vinyl .gamma.-aminobutyric Acid
98. Hsdb 8395
99. Kbio1_001704
100. Kbio2_000848
101. Kbio2_003416
102. Kbio2_005984
103. Kbio3_002973
104. Vigabatrin, (+/-)-
105. Amy6474
106. Vigabatrin [ep Monograph]
107. S(+)-4-aminohexenoicacid
108. Hms1569f03
109. Hms2094m21
110. Hms2096f03
111. Hms3263p16
112. Vigabatrin [usp Monograph]
113. Bcp16220
114. Tox21_110301
115. Tox21_501277
116. Bdbm50118886
117. Akos015854596
118. Ccg-205350
119. Cs-0791
120. Db01080
121. Lp01277
122. Sdccgsbi-0051243.p004
123. .gamma.-vinyl-.gamma.-aminobutyric Acid
124. Ncgc00016087-03
125. Ncgc00016087-04
126. Ncgc00016087-05
127. Ncgc00016087-07
128. Ncgc00016087-08
129. Ncgc00016087-09
130. Ncgc00016087-11
131. Ncgc00016087-22
132. Ncgc00024802-02
133. Ncgc00024802-03
134. Ncgc00024802-04
135. Ncgc00024802-05
136. Ncgc00024802-06
137. Ncgc00261962-01
138. As-11778
139. Hy-15399
140. Sy263247
141. Sbi-0051243.p003
142. ( Inverted Question Mark)-gamma-vinyl Gaba
143. Db-117820
144. Ab00053309
145. Eu-0101277
146. Ft-0675811
147. Ft-0675812
148. Ft-0700968
149. Gamma-vinyl Gaba; 4-amino-5-hexenoic Acid
150. C07500
151. D00535
152. Ab00053309_04
153. A853593
154. Q421663
155. Q-201924
156. Sr-01000075653-1
157. Sr-01000075653-4
158. Sr-01000075653-6
159. ( Inverted Exclamation Marka)-4-aminohex-5-enoic Acid
160. Brd-a07893380-001-01-6
161. Z2235791448
162. Vigabatrin, European Pharmacopoeia (ep) Reference Standard
163. Vigabatrin, United States Pharmacopeia (usp) Reference Standard
164. Vigabatrin Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 129.16 g/mol |
|---|---|
| Molecular Formula | C6H11NO2 |
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 129.078978594 g/mol |
| Monoisotopic Mass | 129.078978594 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 112 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Sabril |
| PubMed Health | Vigabatrin (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | SABRIL (vigabatrin) is an oral antiepileptic drug and is available as white film-coated 500 mg tablets and as a white to off-white granular powder for oral solution in packets of 500 mg. The chemical name of vigabatrin, a racemate consisting of two e... |
| Active Ingredient | Vigabatrin |
| Dosage Form | Tablet; For solution |
| Route | Oral |
| Strength | 500mg; 500mg/packet |
| Market Status | Prescription |
| Company | Lundbeck |
| 2 of 2 | |
|---|---|
| Drug Name | Sabril |
| PubMed Health | Vigabatrin (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | SABRIL (vigabatrin) is an oral antiepileptic drug and is available as white film-coated 500 mg tablets and as a white to off-white granular powder for oral solution in packets of 500 mg. The chemical name of vigabatrin, a racemate consisting of two e... |
| Active Ingredient | Vigabatrin |
| Dosage Form | Tablet; For solution |
| Route | Oral |
| Strength | 500mg; 500mg/packet |
| Market Status | Prescription |
| Company | Lundbeck |
Anticonvulsants; Enzyme Inhibitors; GABA Agents
National Library of Medicine's Medical Subject Headings. Vigabatrin. Online file (MeSH, 2017). Available from, as of Oct 4, 2017: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Vigabatrin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 30, 2017: https://clinicaltrials.gov/
Sabril is indicated as adjunctive therapy for adults and pediatric patients 10 years of age and older with refractory complex partial seizures who have inadequately responded to several alternative treatments and for whom the potential benefits outweigh the risk of vision loss. Sabril is not indicated as a first line agent for complex partial seizures. /Included in US product label/
NIH; DailyMed. Current Medication Information for Sabril (Vigabatrin Tablet, Film Coated) (Updated: April 2017). Available from, as of October 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a5d389d2-d0e1-4395-a2a2-b552808e7f98
Sabril is indicated as monotherapy for pediatric patients with infantile spasms 1 month to 2 years of age for whom the potential benefits outweigh the potential risk of vision loss. /Included in US product label/
NIH; DailyMed. Current Medication Information for Sabril (Vigabatrin Tablet, Film Coated) (Updated: April 2017). Available from, as of October 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a5d389d2-d0e1-4395-a2a2-b552808e7f98
/BOXED WARNING/ WARNING: PERMANENT VISION LOSS. Sabril can cause permanent bilateral concentric visual field constriction, including tunnel vision that can result in disability. In some cases, Sabril also can damage the central retina and may decrease visual acuity. The onset of vision loss from Sabril is unpredictable, and can occur within weeks of starting treatment or sooner, or at any time after starting treatment, even after months or years. Symptoms of vision loss from Sabril are unlikely to be recognized by patients or caregivers before vision loss is severe. Vision loss of milder severity, while often unrecognized by the patient or caregiver, can still adversely affect function. The risk of vision loss increases with increasing dose and cumulative exposure, but there is no dose or exposure known to be free of risk of vision loss. Vision assessment is recommended at baseline (no later than 4 weeks after starting Sabril), at least every 3 months during therapy, and about 3 to 6 months after the discontinuation of therapy. Once detected, vision loss due to Sabril is not reversible. It is expected that, even with frequent monitoring, some patients will develop severe vision loss. Consider drug discontinuation, balancing benefit and risk, if visual loss is documented. Risk of new or worsening vision loss continues as long as Sabril is used. It is possible that vision loss can worsen despite discontinuation of Sabril. Because of the risk of vision loss, Sabril should be withdrawn from patients with refractory complex partial seizures who fail to show substantial clinical benefit within 3 months of initiation and within 2-4 weeks of initiation for patients with infantile spasms, or sooner if treatment failure becomes obvious. Patient response to and continued need for Sabril should be periodically reassessed. Sabril should not be used in patients with, or at high risk of, other types of irreversible vision loss unless the benefits of treatment clearly outweigh the risks. Sabril should not be used with other drugs associated with serious adverse ophthalmic effects such as retinopathy or glaucoma unless the benefits clearly outweigh the risks. Use the lowest dosage and shortest exposure to Sabril consistent with clinical objectives. Because of the risk of permanent vision loss, Sabril is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Vigabatrin REMS Program
NIH; DailyMed. Current Medication Information for Sabril (Vigabatrin Tablet, Film Coated) (Updated: April 2017). Available from, as of October 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a5d389d2-d0e1-4395-a2a2-b552808e7f98
Visual field defects, including permanent vision loss, have been reported in infants, children, and adults receiving vigabatrin. Based on clinical studies in adults, bilateral concentric visual field constriction ranging in severity from mild to severe may occur in 30% or more of patients receiving the drug. Severe cases may be characterized by tunnel vision to within 10 degrees of visual fixation, which can lead to disability. In some cases, vigabatrin can also damage the central retina and decrease visual acuity. Because vision assessment may be difficult in infants and children, the frequency and extent of vision loss is poorly characterized in such patients; therefore, the understanding of the risk is mainly based on adult experience with the drug. The possibility that vigabatrin-induced vision loss may be more common, more severe, or have more functional consequences in infants and children than in adults cannot be excluded.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2420-1
The onset and progression of vision loss with vigabatrin are unpredictable and can occur within weeks of beginning treatment or sooner or at any time after starting therapy, even after months or years. In addition, vision loss may develop or worsen precipitously between vision assessments. Symptoms of vigabatrin-associated vision loss are unlikely to be recognized by patients or caregivers before the impairment is severe. Vision loss of milder severity that is often unrecognized by the patient or caregiver can still adversely affect function. Once detected, vigabatrin-induced visual field defects are irreversible and will not improve even after the drug is discontinued. In addition, it is possible that further impairment of vision may occur following drug discontinuance. Risk of vision loss increases with increasing dosages and cumulative exposure to vigabatrin; however, no dosage or exposure to the drug is known to be free of the risk of vision loss. Some studies have suggested that smoking, age, and male gender are possible risk factors for developing visual field defects.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2421
In patients with infantile spasms, vigabatrin therapy should be withdrawn if a substantial clinical benefit is not observed within 2-4 weeks of initiating the drug. If, in the clinical judgment of the prescribing clinician, evidence of treatment failure becomes obvious earlier than 2-4 weeks, vigabatrin treatment should be discontinued at that time.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2421
For more Drug Warnings (Complete) data for Vigabatrin (25 total), please visit the HSDB record page.
Vigabatrin is indicated as adjunctive therapy in the treatment of refractory complex partial seizures in patients 2 years of age and older who have had inadequate responses to multiple previous treatments (i.e. not to be used for first-line therapy). It is also indicated as monotherapy in the treatment of infantile spasms in patients between 1 month and 2 years of age for whom the potential benefits outweigh the risk of vision loss.
Kigabeq is indicated in infants and children from 1 month to less than 7 years of age for:
- Treatment in monotherapy of infantile spasms (West's syndrome).
- Treatment in combination with other antiepileptic medicinal products for patients with resistant partial epilepsy (focal onset seizures) with or without secondary generalisation, that is where all other appropriate medicinal product combinations have proved inadequate or have not been tolerated.
Vigabatrin is an antiepileptic agent chemically unrelated to other anticonvulsants. Vigabatrin prevents the metabolism of GABA by irreversibly inhibiting GABA transaminase (GABA-T). As vigabatrin is an irreversible inhibitor of gamma-aminobutyric acid transaminase (GABA-T), its duration of effect is thought to be dependent on the rate of GABA-T re-synthesis rather than on the rate of drug elimination.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
GABA Agents
Substances used for their pharmacological actions on GABAergic systems. GABAergic agents include agonists, antagonists, degradation or uptake inhibitors, depleters, precursors, and modulators of receptor function. (See all compounds classified as GABA Agents.)
N03AG04
N03AG04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AG - Fatty acid derivatives
N03AG04 - Vigabatrin
Absorption
Absorption following oral administration is essentially complete. The Tmax is approximately 2.5 hours in infants (5m - 2y) and 1 hour in all other age groups.
Route of Elimination
Approximately 95% of the drug is eliminated in the urine within 72 hours of administration, of which ~80% is unchanged parent drug.
Volume of Distribution
Vigabatrin is widely distributed throughout the body with a mean steady-state volume of distribution of 1.1 L/kg.
Clearance
The oral clearance of vigabatrin is 2.4 L/h for infants (5m - 2y), 5.1 L/h for children (3y - 9y), 5.8 L/h for adolescents (10y - 16y), and 7 L/h for adults.
Drug transporters in various tissues, such as intestine, kidney, liver and brain, are recognized as important mediators of absorption, distribution, metabolism and excretion of drug substances. This review gives a current status on the transporter(s) mediating the absorption, distribution, metabolism and excretion properties of the anti-epileptic drug substance vigabatrin. For orally administered drugs, like vigabatrin, the absorption from the intestine is a prerequisite for the bioavailability. Therefore, transporter(s) involved in the intestinal absorption of vigabatrin in vitro and in vivo are discussed in detail. Special focus is on the contribution of the proton-coupled amino acid transporter 1 (PAT1) for intestinal vigabatrin absorption. Furthermore, the review gives an overview of the pharmacokinetic parameters of vigabatrin across different species and drug-food and drug-drug interactions involving vigabatrin.
PMID:25337649 Nohr MK et al; Ther Deliv 5 (8): 927-42 (2014)
The aims were to determine blood-brain barrier penetration and brain extracellular pharmacokinetics for the anticonvulsant vigabatrin (VGB; gamma-vinyl-gamma-aminobutyric acid) in brain extracellular fluid and plasma from severe traumatic brain injury (TBI) patients, and to measure the response of gamma-aminobutyric acid (GABA) concentration in brain extracellular fluid. Severe TBI patients (n = 10) received VGB (0.5 g enterally, every 12 hr). Each patient had a cerebral microdialysis catheter; two patients had a second catheter in a different region of the brain. Plasma samples were collected 0.5 hr before and 2, 4 and 11.5 hr after the first VGB dose. Cerebral microdialysis commenced before the first VGB dose and continued through at least three doses of VGB. Controls were seven severe TBI patients with microdialysis, without VGB. After the first VGB dose, the maximum concentration of VGB (Cmax) was 31.7 (26.9-42.6) umol/L (median and interquartile range for eight patients) in plasma and 2.41 (2.03-5.94) umol/L in brain microdialysates (nine patients, 11 catheters), without significant plasma-brain correlation. After three doses, median Cmax in microdialysates increased to 5.22 (4.24-7.14) umol/L (eight patients, 10 catheters). Microdialysate VGB concentrations were higher close to focal lesions than in distant sites. Microdialysate GABA concentrations increased modestly in some of the patients after VGB administration. Vigabatrin, given enterally to severe TBI patients, crosses the blood-brain barrier into the brain extracellular fluid, where it accumulates with multiple dosing. Pharmacokinetics suggest delayed uptake from the blood.
PMID:24802902 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4243872 Shannon RJ et al; Br J Clin Pharmacol 78 (5): 981-95 (2014)
/MILK/ Vigabatrin distributes into milk, probably in small amounts.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2422
The aim of the study was to investigate the intestinal transport mechanisms responsible for vigabatrin absorption in rats by developing a population pharmacokinetic (PK) model of vigabatrin oral absorption. The PK model was used to investigate whether vigabatrin absorption was carrier-mediated and if the proton-coupled amino acid transporter 1 (PAT1) was involved in the absorption processes. Vigabatrin (0.3-300 mg/kg) was administered orally or intravenously to Sprague Dawley rats in the absence or presence of PAT1-ligands l-proline, l-tryptophan or sarcosine. The PK profiles of vigabatrin were described by mechanistic non-linear mixed effects modelling, evaluating PAT1-ligands as covariates on the PK parameters with a full covariate modelling approach. The oral absorption of vigabatrin was adequately described by a Michaelis-Menten type saturable absorption. Using a Michaelis constant of 32.8 mM, the model estimated a maximal oral absorption rate (Vmax) of 64.6mmol/min and dose-dependent bioavailability with a maximum of 60.9%. Bioavailability was 58.5-60.8% at 0.3-30 mg/kg doses, but decreased to 46.8% at 300 mg/kg. Changes in oral vigabatrin PK after co-administration with PAT1-ligands was explained by significant increases in the apparent Michaelis constant. Based on the mechanistic model, a high capacity low affinity carrier is proposed to be involved in intestinal vigabatrin absorption. PAT1-ligands increased the Michaelis constant of vigabatrin after oral co-administration indicating that this carrier could be PAT1.
PMID:25562534 Nohr MK et al; Eur J Pharm Sci 69:10-8 (2015)
For more Absorption, Distribution and Excretion (Complete) data for Vigabatrin (10 total), please visit the HSDB record page.
Vigabatrin is not metabolized to any significant extent.
Vigabatrin is not significantly metabolized ... .
NIH; DailyMed. Current Medication Information for Sabril (Vigabatrin Tablet, Film Coated) (Updated: April 2017). Available from, as of October 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a5d389d2-d0e1-4395-a2a2-b552808e7f98
The terminal half-life of vigabatrin is approximately 5.7 hours for infants (5m - 2y), 6.8 hours for children (3y - 9y), 9.5 hours for adolescents (10y - 16y), and 10.5 h for adults.
The terminal half-life of vigabatrin is about 5.7 hours for infants (5 months - 2 years), 9.5 hours for children (10 years - 16 years), and 10.5 hours for adults.
NIH; DailyMed. Current Medication Information for Sabril (Vigabatrin Tablet, Film Coated) (Updated: April 2017). Available from, as of October 19, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a5d389d2-d0e1-4395-a2a2-b552808e7f98
Gamma-aminobutyric acid (GABA) is the major inhibitory transmitter throughout the central nervous system, and the potentiation of GABAergic neurotransmission is therefore a crucial mechanism through which antiepileptic agents may combat the pathologic excitatory neurotransmission seen in epilepsy. Vigabatrin increases concentrations of GABA in the central nervous system by irreversibly inhibiting the enzymes responsible for its metabolism to succinic semialdehyde: gamma-aminobutyric acid transaminase (GABA-T).
Vigabatrin is a structural analog of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the CNS. Although the exact mechanism of vigabatrin's antiseizure effect is unknown, it is thought to be related to the drug's action as a preferential and irreversible inhibitor of GABA transaminase (GABA-T), which is the enzyme responsible for the degradation of GABA and the resultant increase in GABA concentrations in the CNS. Vigabatrin is commercially available as a racemic mixture of 2 enantiomers; the S enantiomer is pharmacologically active and the R enantiomer is inactive.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2423
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
About the Company : Biophore, founded in 2007, develops and manufactures niche and complex pharmaceutical products. With USFDA- and EU-approved API facilities, a dedicated intermediates site and an R&...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
About the Company : HRV Pharma is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
About the Company : Granules is a fast-growing, vertically integrated pharma company based in Hyderabad, committed to operational excellence, quality, and customer service. It manufactures across the ...
 Lewens Labs transforms healthcare with innovative, sustainable, and affordable pharmaceutical solutions worldwide.
Lewens Labs transforms healthcare with innovative, sustainable, and affordable pharmaceutical solutions worldwide.
About the Company : Lewens Labs was established with a vision to transform the pharmaceutical industry, inspired by an award-winning engineering project that reduced the cost of Aceclofenac and earned...
About the Company : Founded in 1986 by Mr. P.V. Ramaprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born off a vision. The company comme...

About the Company : Divis Laboratories Limited is a leading independent contract manufacturer of Active Pharmaceutical Ingredients and registered intermediates. We custom / exclusively manufacture for...

About the Company : Hy-Gro Chemicals Pharmtek Pvt. Ltd. (Hy-Gro) is a fast growing pharmaceutical company engaged in the manufacture of Active Pharmaceutical Ingredients (APIs), Advanced Intermediates...

About the Company : Wuhan Sun-shine chemical Corporation Limited (Shanghai Sun-shine Chemical Technology Corporation Limited) is a high-tech enterprise engaged inR&D and sales of related compounds of ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2042-10-17
US Patent Number : 12290499
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 217684
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2042-10-17

Patent Expiration Date : 2039-08-16
US Patent Number : 12016857
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 217684
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2039-08-16

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
11
PharmaCompass offers a list of Vigabatrin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vigabatrin manufacturer or Vigabatrin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vigabatrin manufacturer or Vigabatrin supplier.
PharmaCompass also assists you with knowing the Vigabatrin API Price utilized in the formulation of products. Vigabatrin API Price is not always fixed or binding as the Vigabatrin Price is obtained through a variety of data sources. The Vigabatrin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vigabatrin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vigabatrin, including repackagers and relabelers. The FDA regulates Vigabatrin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vigabatrin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vigabatrin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vigabatrin supplier is an individual or a company that provides Vigabatrin active pharmaceutical ingredient (API) or Vigabatrin finished formulations upon request. The Vigabatrin suppliers may include Vigabatrin API manufacturers, exporters, distributors and traders.
click here to find a list of Vigabatrin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Vigabatrin DMF (Drug Master File) is a document detailing the whole manufacturing process of Vigabatrin active pharmaceutical ingredient (API) in detail. Different forms of Vigabatrin DMFs exist exist since differing nations have different regulations, such as Vigabatrin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Vigabatrin DMF submitted to regulatory agencies in the US is known as a USDMF. Vigabatrin USDMF includes data on Vigabatrin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Vigabatrin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Vigabatrin suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Vigabatrin Drug Master File in Korea (Vigabatrin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Vigabatrin. The MFDS reviews the Vigabatrin KDMF as part of the drug registration process and uses the information provided in the Vigabatrin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Vigabatrin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Vigabatrin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Vigabatrin suppliers with KDMF on PharmaCompass.
A Vigabatrin CEP of the European Pharmacopoeia monograph is often referred to as a Vigabatrin Certificate of Suitability (COS). The purpose of a Vigabatrin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Vigabatrin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Vigabatrin to their clients by showing that a Vigabatrin CEP has been issued for it. The manufacturer submits a Vigabatrin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Vigabatrin CEP holder for the record. Additionally, the data presented in the Vigabatrin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Vigabatrin DMF.
A Vigabatrin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Vigabatrin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Vigabatrin suppliers with CEP (COS) on PharmaCompass.
A Vigabatrin written confirmation (Vigabatrin WC) is an official document issued by a regulatory agency to a Vigabatrin manufacturer, verifying that the manufacturing facility of a Vigabatrin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Vigabatrin APIs or Vigabatrin finished pharmaceutical products to another nation, regulatory agencies frequently require a Vigabatrin WC (written confirmation) as part of the regulatory process.
click here to find a list of Vigabatrin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Vigabatrin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Vigabatrin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Vigabatrin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Vigabatrin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Vigabatrin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Vigabatrin suppliers with NDC on PharmaCompass.
Vigabatrin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vigabatrin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vigabatrin GMP manufacturer or Vigabatrin GMP API supplier for your needs.
A Vigabatrin CoA (Certificate of Analysis) is a formal document that attests to Vigabatrin's compliance with Vigabatrin specifications and serves as a tool for batch-level quality control.
Vigabatrin CoA mostly includes findings from lab analyses of a specific batch. For each Vigabatrin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vigabatrin may be tested according to a variety of international standards, such as European Pharmacopoeia (Vigabatrin EP), Vigabatrin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vigabatrin USP).

