
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Erivedge
2. Gdc 0449
3. Gdc-0449
4. Gdc0449
5. Hhantag691
6. Nsc 747691
7. Nsc-747691
8. Nsc747691
9. R 3616
10. R-3616
11. R3616 Cpd
12. Rg 3616
13. Rg-3616
14. Rg3616
1. 879085-55-9
2. Gdc-0449
3. Erivedge
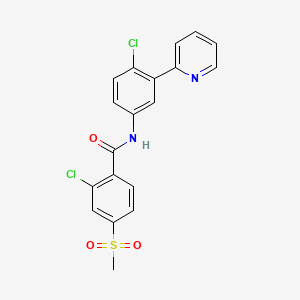
4. 2-chloro-n-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide
5. Vismodegib (gdc-0449)
6. Hhantag691
7. Gdc0449
8. Gdc 0449
9. Gdc-0449 (vismodegib)
10. Nsc-747691
11. Nsc-755986
12. 2-chloro-n-[4-chloro-3-(pyridin-2-yl)phenyl]-4-(methylsulfonyl)benzamide
13. Chembl473417
14. Chebi:66903
15. 2-chloro-n-(4-chloro-3-pyridin-2-ylphenyl)-4-methylsulfonylbenzamide
16. 25x868m3ds
17. Nsc755986
18. 2-chloro-n-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)benzamide
19. 2-chloro-n-(4-chloro-3-(2-pyridinyl)phenyl)-4-(methylsulfonyl)benzamide
20. 2-chloro-n-(4-chloro-3-pyridin-2-yl-phenyl)-4-methanesulfonyl-benzamide
21. 2-chloro-n-[4-chloro-3-(pyridin-2-yl)phenyl]-4-methanesulfonylbenzamide
22. Benzamide, 2-chloro-n-(4-chloro-3-(2-pyridinyl)phenyl)-4-(methylsulfonyl)-
23. 2-chloranyl-~{n}-(4-chloranyl-3-pyridin-2-yl-phenyl)-4-methylsulfonyl-benzamide
24. Erivedge (tn)
25. Vismodegib [usan]
26. Vismodegib [usan:inn]
27. Nsc 747691
28. Vismodegibum
29. Unii-25x868m3ds
30. Hsdb 8130
31. R 3616
32. Nsc747691
33. Hh-antag691
34. Hhantag 691
35. Vismodegib, Free Base
36. Rg 3616
37. Rg-3616
38. Methanesulfonylbenzamide
39. Vismodegib [mi]
40. Vismodegib [inn]
41. Vismodegib (usan/inn)
42. Vismodegib [vandf]
43. Vismodegib; Gdc-0449
44. R-3616
45. Vismodegib [who-dd]
46. Gdc-0449 - Selumetinib
47. Mls006012035
48. Schembl302587
49. Gtpl6975
50. Vismodegib [orange Book]
51. Cur-691
52. Dtxsid40236689
53. Gdc-449
54. Bcpp000223
55. Hms3604k16
56. Hms3654e17
57. Bcp01715
58. Ex-a2178
59. Bdbm50249522
60. Mfcd12407408
61. Nsc755809
62. S1082
63. Zinc40899447
64. Gdc-0449,vismodegib, Hhantag691
65. 2-chloro-n-(4-chloro-3-(pyridin-2-yl)-phenyl)-4-(methylsulfonyl)benzamide
66. Akos015966534
67. Bcp9000713
68. Ccg-264811
69. Cs-0255
70. Db08828
71. Nsc-755809
72. Pb15086
73. Ncgc00242497-01
74. Ncgc00242497-02
75. Ncgc00242497-06
76. Ncgc00242497-12
77. Ac-26969
78. As-14066
79. Hy-10440
80. Smr004703564
81. Ft-0675833
82. Sw218087-2
83. Ec-000.2333
84. D09992
85. V-4050
86. Ab01565813_02
87. 085g559
88. Sr-01000941574
89. 2-chloro-n-[4-chloro-3-(pyridin-2-yl)phenyl]-4-
90. J-509076
91. Q2070286
92. Sr-01000941574-1
93. C538724000
94. 2-chloro-n-(4-chloro-3-(2-pyridl)phenyl)-4-(methylsulfonyl)benzamide
95. 2-chloro-n-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methyl Sulfonyl)benzamide
96. 2-chloro-n~1~-[4-chloro-3-(2-pyridyl)phenyl]-4-(methylsulfonyl)benzamide
97. 2-chloro-n-(4-chloro-3-pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide
98. 2-chloro-n-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)-benzamide
99. Vis
| Molecular Weight | 421.3 g/mol |
|---|---|
| Molecular Formula | C19H14Cl2N2O3S |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 420.0102189 g/mol |
| Monoisotopic Mass | 420.0102189 g/mol |
| Topological Polar Surface Area | 84.5 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 625 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Erivedge |
| PubMed Health | Vismodegib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Vismodegib is an inhibitor of the hedgehog (Hh) signaling pathway, which is described chemically as 2-Chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide. The molecular formula is C19H14Cl2N2O3S. The molecular weight is 421.30 g/mo... |
| Active Ingredient | Vismodegib |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Genentech |
| 2 of 2 | |
|---|---|
| Drug Name | Erivedge |
| PubMed Health | Vismodegib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Vismodegib is an inhibitor of the hedgehog (Hh) signaling pathway, which is described chemically as 2-Chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide. The molecular formula is C19H14Cl2N2O3S. The molecular weight is 421.30 g/mo... |
| Active Ingredient | Vismodegib |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Genentech |
Erivedge capsule is indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
/BOXED WARNING/ WARNING: EMBRYO-FETAL DEATH AND SEVERE BIRTH DEFECTS ERIVEDGE (vismodegib) capsule can result in embryo-fetal death or severe birth defects. Erivedge is embryotoxic and teratogenic in animals. Teratogenic effects included severe midline defects, missing digits, and other irreversible malformations. Verify pregnancy status prior to the initiation of Erivedge. Advise male and female patients of these risks. Advise female patients of the need for contraception and advise male patients of the potential risk of Erivedge exposure through semen.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
Advise patients not to donate blood or blood products while receiving Erivedge and for at least 7 months after the last dose of Erivedge.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
Dysregulated hedgehog signaling is the pivotal molecular abnormality underlying basal-cell carcinomas. Vismodegib is a new orally administered hedgehog-pathway inhibitor that produces objective responses in locally advanced and metastatic basal-cell carcinomas. /The researchers/ tested the anti-basal-cell carcinoma efficacy of vismodegib in a randomized, double-blind, placebo-controlled trial in patients with the basal-cell nevus syndrome at three clinical centers from September 2009 through January 2011. The primary end point was reduction in the incidence of new basal-cell carcinomas that were eligible for surgical resection (surgically eligible) with vismodegib versus placebo after 3 months; secondary end points included reduction in the size of existing basal-cell carcinomas. In 41 patients followed for a mean of 8 months (range, 1 to 15) after enrollment, the per-patient rate of new surgically eligible basal-cell carcinomas was lower with vismodegib than with placebo (2 vs. 29 cases per group per year, P<0.001), as was the size (percent change from baseline in the sum of the longest diameter) of existing clinically significant basal-cell carcinomas (-65% vs. -11%, P=0.003). In some patients, all basal-cell carcinomas clinically regressed. No tumors progressed during treatment with vismodegib. Patients receiving vismodegib routinely had grade 1 or 2 adverse events of loss of taste, muscle cramps, hair loss, and weight loss. Overall, 54% of patients (14 of 26) receiving vismodegib discontinued drug treatment owing to adverse events. At 1 month, vismodegib use had reduced the hedgehog target-gene expression by basal-cell carcinoma by 90% (P<0.001) and diminished tumor-cell proliferation, but apoptosis was not affected. No residual basal-cell carcinoma was detectable in 83% of biopsy samples taken from sites of clinically regressed basal-cell carcinomas. Vismodegib reduces the basal-cell carcinoma tumor burden and blocks growth of new basal-cell carcinomas in patients with the basal-cell nevus syndrome. The adverse events associated with treatment led to discontinuation in over half of treated patients. Comment in The following popper user interface control may not be accessible. Tab to the next button to revert the control to an accessible version. Destroy user interface controlVismodegib in advanced basal-cell carcinoma.
PMID:22670904 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4362529 Tang JY et al; N Engl J Med 366 (23): 2180-8 (2012)
FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
For more Drug Warnings (Complete) data for Vismodegib (8 total), please visit the HSDB record page.
Vismodegib is used for treating locally advanced or metastatic basal cell carcinoma in patients whose carcinoma has recurred after surgery, and in patients who are not candidates for surgery or radiation.
FDA Label
Erivedge is indicated for the treatment of adult patients with:
- symptomatic metastatic basal cell carcinoma
- locally advanced basal cell carcinoma inappropriate for surgery or radiotherapy
Vismodegib selectively binds to and inhibits the transmembrane protein Smoothened homologue (SMO) to inhibit the Hedgehog signalling pathway.
L01XX43
L01XX43
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XJ - Hedgehog pathway inhibitors
L01XJ01 - Vismodegib
Absorption
The absolute bioavailability of a single dose is 31.8%. Absorption is saturable and is not affected by food.
Route of Elimination
Vismodegib is mostly excreted unchanged, and the main route of elimination is by the feces (82%) and the urine accounts for 4.4%.
Volume of Distribution
Vismodegib has a volume of distribution of 16.4 to 26.6 L.
The volume of distribution of vismodegib ranges from 16.4 to 26.6 L. Vismodegib plasma protein binding in patients is greater than 99%. Vismodegib binds to both human serum albumin and alpha-1-acid glycoprotein (AAG) and binding to AAG is saturable.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
The single dose absolute bioavailability of vismodegib is 31.8%. Absorption is saturable as evidenced by the lack of dose proportional increase in exposure after a single dose of 270 mg or 540 mg vismodegib. Erivedge capsule may be taken without regard to meals because the systemic exposure of vismodegib at steady state is not affected by food.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
Vismodegib and its metabolites are eliminated primarily by the hepatic route with 82% of the administered dose recovered in the feces and 4.4% recovered in urine.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
While recent publications have suggested the pharmacokinetics (PK) of vismodegib appear to be non-linear, there has not been a report describing the mechanisms of non-linearity. This study provides evidence that two separate processes, namely, solubility-limited absorption and concentration-dependent plasma protein binding, can explain the non-linear PK of vismodegib. This study provides quantitative results which can account for the lower than expected accumulation of vismodegib with continuous daily dosing. Vismodegib has demonstrated clinical activity in patients with advanced basal cell carcinoma. The pharmacokinetics (PK) of vismodegib are non-linear. The objective of this study was to determine whether vismodegib PK change following repeated dosing by administering a tracer intravenous (iv) dose of (14) C-vismodegib with single and multiple oral doses. Healthy post menopausal female subjects (n= 6/group) received either a single or daily 150 mg vismodegib oral dose with a (14) C-labelled 10 ug iv bolus dose administered 2 hr after the single or last oral dose (day 7). Plasma samples were assayed for vismodegib by LC-MS/MS and for (14) C-vismodegib by accelerator mass spectrometry. Following a single i.v. dose, mean clearance, volume of distribution and absolute bioavailability were 43.4 mL hr(-1) , 16.4 l and 31.8%, respectively. Parallel concentration-time profiles following single oral and i.v. administration of vismodegib indicated elimination rate limited PK. Following iv administration at steady-state, mean clearance and volume of distribution were 78.5 mL hr(-1) and 26.8 L, respectively. Comparison of iv PK parameters after single and multiple oral dosing showed similar half-life, increased clearance and volume of distribution (81% and 63% higher, respectively) and decreased bioavailability (77% lower) after repeated dosing. Relative to single dose, the unbound fraction of vismodegib increased 2.4-fold with continuous daily dosing. Vismodegib exhibited a long terminal half-life after oral and iv administration, moderate absolute bioavailability and non-linear PK after repeated dosing. Results from this study suggest that the non-linear PK of vismodegib result from two separate, non-linear processes, namely solubility limited absorption and high affinity, saturable plasma protein binding.
PMID:22458643 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3495143 Graham RA et al; Br J Clin Pharmacol 74 (5): 788-96 (2012)
For more Absorption, Distribution and Excretion (Complete) data for Vismodegib (7 total), please visit the HSDB record page.
The main metabolic enzymes are CYP2C9 and CYP3A4, however more than 98% of total systemic vismodegib is not metabolized. Metabolic pathways of vismodegib in humans include oxidation, glucuronidation, and pyridine ring cleavage. The two most abundant oxidative metabolites recovered in feces are produced in vitro by recombinant CYP2C9 and CYP3A4/5.
Greater than 98% of the total circulating drug-related components are the parent drug. Metabolic pathways of vismodegib in humans include oxidation, glucuronidation, and pyridine ring cleavage. The two most abundant oxidative metabolites recovered in feces are produced in vitro by recombinant CYP2C9 and CYP3A4/5.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
2-Chloro-N-(4-chloro-3-(pyridin-2-yl)-phenyl)-4-(methylsulfonyl)-benzamide (GDC-0449, vismodegib) is a potent and selective first-in-class small-molecule inhibitor of the Hedgehog signaling pathway and is currently in clinical development. In this study, we investigated the metabolic fate and disposition of GDC-0449 in rats and dogs after a single oral administration of (14)C-GDC-0449. ... GDC-0449 underwent extensive metabolism in rats and dogs with the major metabolic pathways being oxidation of the 4-chloro-3-(pyridin-2-yl)-phenyl moiety followed by phase II glucuronidation or sulfation. Three other metabolites resulting from an uncommon pyridine ring opening were found, mainly in feces, representing 1.7 to 17.7% of the dose in total in rats and dogs. ...
PMID:21363998 Yue Q et al; Drug Metab Dispos 39 (6): 952-65 (2011)
... Proposed metabolites from exploratory metabolite identification in vitro (rat, dog and human liver microsomes) and in vivo (dog and rat urine) include three primary oxidative metabolites (M1-M3) and three sequential glucuronides (M4-M6). Oxidative metabolites identified in microsomes M1 and M3 were formed primarily by P4503A4/5 (M1) and P4502C9 (M3). GDC-0449 was not a potent inhibitor of P4501A2, P4502B6, P4502D6, and P4503A4/5 with IC50 estimates greater than 20 uM. K(i)'s estimated for P4502C8, P4502C9 and P4502C19 and were 6.0, 5.4 and 24 uM, respectively. An evaluation with Simcyp suggests that GDC-0449 has a low potential of inhibiting P4502C8 and P4502C9. Furthermore, GDC-0449 (15 uM) was not a potent P-glycoprotein/ABCB1 inhibitor in MDR1-MDCK cells.
PMID:19845436 Wong H et al; Xenobiotica 39 (11): 850-61 (2009)
The half-life after a single dose is 12 days, and after continuous daily dosing is 4 days.
The estimated elimination half-life of vismodegib is 4 days after continuous once-daily dosing and 12 days after a single dose.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
Mutations of the Hedgehog pathway may results in uncontrolled proliferation of skin basal cells. Vismodegib binds to and inhibits the transmembrane protein Smoothened homologue (SMO) to inhibit the Hedgehog signalling pathway.
Vismodegib is an inhibitor of the Hedgehog pathway. Vismodegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction.
US Natl Inst Health; DailyMed. Current Medication Information for ERIVEDGE (vismodegib) capsule (January 2012). Available from, as of March 11, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=eb368bb6-80e3-4df9-8a85-91df0a2ada6a
Recent evidence from in vitro and in vivo studies has demonstrated that aberrant reactivation of the Sonic Hedgehog (SHH) signaling pathway regulates genes that promote cellular proliferation in various human cancer stem cells (CSCs). Therefore, the chemotherapeutic agents that inhibit activation of Gli transcription factors have emerged as promising novel therapeutic drugs for pancreatic cancer. GDC-0449 (Vismodegib), orally administrable molecule belonging to the 2-arylpyridine class, inhibits SHH signaling pathway by blocking the activities of Smoothened. The objectives of this study were to examine the molecular mechanisms by which GDC-0449 regulates human pancreatic CSC characteristics in vitro. GDC-0499 inhibited cell viability and induced apoptosis in three pancreatic cancer cell lines and pancreatic CSCs. This inhibitor also suppressed cell viability, Gli-DNA binding and transcriptional activities, and induced apoptosis through caspase-3 activation and PARP cleavage in pancreatic CSCs. GDC-0449-induced apoptosis in CSCs showed increased Fas expression and decreased expression of PDGFRa. Furthermore, Bcl-2 was down-regulated whereas TRAIL-R1/DR4 and TRAIL-R2/DR5 expression was increased following the treatment of CSCs with GDC-0449. Suppression of both Gli1 plus Gli2 by shRNA mimicked the changes in cell viability, spheroid formation, apoptosis and gene expression observed in GDC-0449-treated pancreatic CSCs. Thus, activated Gli genes repress DRs and Fas expressions, up-regulate the expressions of Bcl-2 and PDGFRa and facilitate cell survival. These data suggest that GDC-0499 can be used for the management of pancreatic cancer by targeting pancreatic CSCs.
PMID:22087285 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3210776 Singh BN et al; PLoS One. 2011;6(11):e27306. doi: 10.1371/journal.pone.0027306. Epub 2011
Hedgehog (Hh) signaling is involved in the pathogenesis of liver fibrosis. It has been previously shown that Hh-inhibitor cyclopamine (CYA) can reduce liver fibrosis in rats. However, CYA is not stable in vivo, which limits its clinical application. This study compares the antifibrotic potential of two known Hh antagonists, vismodegib (GDC-0449, abbreviated to GDC) and CYA. GDC is a synthetic molecule presently in clinical cancer trials and has been reported to be safe and efficacious. These drugs attenuated early liver fibrosis in common bile duct ligated rats, improved liver function, and prevented hepatic stellate cell (HSC) activation, thereby suppressing epithelial to mesenchymal transition (EMT). While both CYA and GDC increased the number of proliferating cell nuclear antigen positive liver cells in vivo, only CYA increased Caspase-3 expression in HSCs in rat livers, suggesting that while GDC and CYA effectively attenuate early liver fibrosis, their hepatoprotective effects may be mediated through different modes of action. Thus, GDC has the potential to serve as a new therapeutic agent for treating early liver fibrosis.
PMID:22994359 Pratap A et al; J Drug Target 20 (9): 770-82 (2012)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
86
PharmaCompass offers a list of Vismodegib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vismodegib manufacturer or Vismodegib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vismodegib manufacturer or Vismodegib supplier.
PharmaCompass also assists you with knowing the Vismodegib API Price utilized in the formulation of products. Vismodegib API Price is not always fixed or binding as the Vismodegib Price is obtained through a variety of data sources. The Vismodegib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vismodegib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vismodegib, including repackagers and relabelers. The FDA regulates Vismodegib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vismodegib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vismodegib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vismodegib supplier is an individual or a company that provides Vismodegib active pharmaceutical ingredient (API) or Vismodegib finished formulations upon request. The Vismodegib suppliers may include Vismodegib API manufacturers, exporters, distributors and traders.
click here to find a list of Vismodegib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Vismodegib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Vismodegib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Vismodegib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Vismodegib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Vismodegib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Vismodegib suppliers with NDC on PharmaCompass.
Vismodegib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vismodegib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vismodegib GMP manufacturer or Vismodegib GMP API supplier for your needs.
A Vismodegib CoA (Certificate of Analysis) is a formal document that attests to Vismodegib's compliance with Vismodegib specifications and serves as a tool for batch-level quality control.
Vismodegib CoA mostly includes findings from lab analyses of a specific batch. For each Vismodegib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vismodegib may be tested according to a variety of international standards, such as European Pharmacopoeia (Vismodegib EP), Vismodegib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vismodegib USP).
