
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
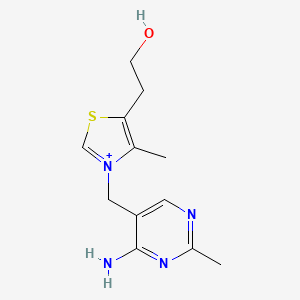

1. Aneurin
2. Mononitrate, Thiamine
3. Thiamin
4. Thiamine Mononitrate
5. Vitamin B 1
6. Vitamin B1
1. Thiamin
2. Vitamin B1
3. Aneurin
4. Thiamine Ion
5. Antiberiberi Factor
6. Thiadoxine
7. Betaxin
8. 70-16-6
9. Biamine
10. Bequin
11. Thiaminium
12. Thiamine(1+)
13. Thiamine(1+) Ion
14. 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium
15. 2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol
16. Thiamine (vit B1)
17. 4abt0j945j
18. Chebi:18385
19. 3-(4-amino-2-methyl-pyrimidin-5-ylmethyl)-5-(2-hydroxy-ethyl)-4-methyl-thiazol-3-ium
20. Vib
21. Thd
22. 3-((4-amino-2-methylpyrimidin-5-yl)methyl)-5-(2-hydroxyethyl)-4-methylthiazol-3-ium
23. Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-5-(2-hydroxyethyl)-4-methyl-
24. [3h]thiamine
25. [3h]-thiamine
26. Vitamin B 1
27. Nsc36226
28. [3h]vitamin B1
29. 1sbr
30. 3rlb
31. Thoh
32. Prestwick0_000631
33. Prestwick1_000631
34. Prestwick2_000631
35. Prestwick3_000631
36. Bmse000274
37. Timtec1_000613
38. Chembl1547
39. Thiamin; Vitamin B1
40. Unii-4abt0j945j
41. Schembl10075
42. Bspbio_000622
43. Spbio_002841
44. Bpbio1_000686
45. Gtpl4628
46. Gtpl4629
47. Schembl22129283
48. Zinc49153
49. Dtxsid50220251
50. Bdbm50373877
51. Stl301841
52. Akos000668650
53. Db00152
54. 2-[3-[(4-amino-2-methyl-pyrimidin-5-yl)methyl]-4-methyl-thiazol-3-ium-5-yl]ethanol
55. 3[(4-amino-2-methyl-5-pyrimidinyl)-methyl]-5-(2-hydroxyethyl)-4-methylthiazolium Chloride
56. Smp1_000084
57. Ncgc00017013-06
58. Ncgc00188957-01
59. Ncgc00188957-02
60. C00378
61. Q83187
62. 3-(2-methyl-4-aminopyrimidine-5-ylmethyl)-4-methylthiazolium-5-ethanol
| Molecular Weight | 265.36 g/mol |
|---|---|
| Molecular Formula | C12H17N4OS+ |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 265.11230735 g/mol |
| Monoisotopic Mass | 265.11230735 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 1 |
| Complexity | 269 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Thiamine is used to prevent and to treat thiamine deficiency syndromes including beriberi, Wernicke's encephalopathy syndrome, delirium, and peripheral neuritis associated with pellagra or neuritis of pregnancy (if associated with severe vomiting).
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Although thiamine has not been shown by well-controlled trials to have any therapeutic value, the drug has been used for the management of poor appetite, ulcerative colitis, chronic diarrhea, other GI disorders, and the cerebellar syndrome. Thiamine has also been used orally as an insect repellent, but there is a lack of adequate evidence to establish the efficacy of thiamine for this use.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
Low plasma thiamine concentrations have been found in patients with type 1 and type 2 diabetes mellitus. In a small placebo-controlled study, benfotiamine /a related vitamin B1 substance/ 100 mg given four times daily by mouth significantly improved neuropathic pain in patients with diabetic polyneuropathy. /Benfotiamine/
Sweetman SC (ed), Martindale: The Complete Drug Reference. London: Pharmaceutical Press (2009), p.1976-7.
/This study assessed/ the effect of thiamine repletion on thiamine status, functional capacity, and left ventricular ejection fraction (LVEF) in patients with moderate to severe congestive heart failure (CHF) who had received furosemide in doses of 80 mg/d or more for at least 3 months. PATIENTS AND METHODS: Thirty patients were randomized to 1 week of double-blind inpatient therapy with either iv thiamine 200 mg/d or placebo (n = 15 each). All previous drugs were continued. Following discharge, all 30 patients received oral thiamine 200 mg/d as outpatients for 6 weeks. Thiamine status was determined by the erythrocyte thiamine-pyrophosphate effect (TPPE). LVEF was determined by echocardiography. RESULTS: TPPE, diuresis, and LVEF were unchanged with iv placebo. After iv thiamine, TPPE decreased (11.7% +/- 6.5% to 5.4% +/- 3.2%; P < 0.01). LVEF increased (0.28 +/- 0.11 to 0.32 +/- 0.09; P < 0.05), as did diuresis (1,731 +/- 800 mL/d to 2,389 +/- 752 mL/d; P < 0.02), and sodium excretion (84 +/- 52 mEq/d to 116 +/- 83 mEq/d, P < 0.05). In the 27 patients completing the full 7-week intervention, LVEF rose by 22% (0.27 +/- 0.10 to 0.33 +/- 0.11, P < 0.01). CONCLUSIONS: Thiamine repletion can improve left ventricular function and biochemical evidence of thiamine deficiency in some patients with moderate-to-severe CHF who are receiving longterm furosemide therapy.
PMID:7733128 Shimon I et al; Am J Med 98 (5): 485-90 (1995).
For more Therapeutic Uses (Complete) data for Vitamin B1 (11 total), please visit the HSDB record page.
Serious hypersensitivity/anaphylactic reactions can occur, especially after repeated administration. Deaths have resulted from IV or IM administration of thiamine.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 18
Anaphylaxis as an adverse systemic reaction to thiamine (vitamin B1) has been described in the literature since 1938. Although its precise mechanism is still uncertain, the reaction appears to involve immediate type hypersensitivity and to be exclusively related to parenteral administration...
PMID:9846348 Morinville V et al; Schweiz Med Wochenschr 128 (44): 1743-4 (1998).
Anaphylaxis. There have been occasional reports of serious and even fatal responses to the parenteral administration of thiamin. The clinical characteristics have strongly suggested an anaphylactic reaction. Symptoms associated with thiamin-induced anaphylaxis include anxiety, pruritus, respiratory distress, nausea, abdominal pain, and shock, sometimes progressing to death.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press, Washington, D.C., pg. 81, 1998. Available from, as of March 2, 2010: https://www.nap.edu/catalog/6015.html
Adverse reactions with thiamine are rare, but hypersensitivity reactions have occurred, mainly after parenteral doses. These reactions have ranged in severity from very mild to, very rarely, fatal anaphylactic shock ... The UK Committee on Safety of Medicines had received, between 1970 and July, 1988, 90 reports of adverse reactions associated with the use of an injection containing high doses of vitamins B and C. The most frequent reactions were anaphylaxis (41 cases, including 2 fatalities), dyspnea or bronchospasm (13 cases), and rash or flushing (22 cases); 78 of the reactions occurred during, or shortly after, intravenous injection and the other 12 after intramuscular injectdion. They recommended that parenteral treatment be used only when essential, and that, when given, facilities for treating anaphylaxis should be available. They also recommended that, when the intravenous route was used, the injection be given slowly (over 10 minutes). Various authors have noted that parenteral treatment is essential for the prophylaxis and treatment of Wernicke's encephalopathy. However, further reports of anaphylaxis to parenteral thiamine have since been described, including one with a fatal outcome.
Sweetman SC (ed), Martindale: The Complete Drug Reference. London: Pharmaceutical Press (2009), p.1976.
For more Drug Warnings (Complete) data for Vitamin B1 (15 total), please visit the HSDB record page.
For the treatment of thiamine and niacin deficiency states, Korsakov's alcoholic psychosis, Wernicke-Korsakov syndrome, delirium, and peripheral neuritis.
Thiamine is a vitamin with antioxidant, erythropoietic, cognition-and mood-modulatory, antiatherosclerotic, putative ergogenic, and detoxification activities. Thiamine has been found to protect against lead-induced lipid peroxidation in rat liver and kidney. Thiamine deficiency results in selective neuronal death in animal models. The neuronal death is associated with increased free radical production, suggesting that oxidative stress may play an important early role in brain damage associated with thiamine deficiency. Thiamine plays a key role in intracellular glucose metabolism and it is thought that thiamine inhibits the effect of glucose and insulin on arterial smooth muscle cell proliferation. Inhibition of endothelial cell proliferation may also promote atherosclerosis. Endothelial cells in culture have been found to have a decreased proliferative rate and delayed migration in response to hyperglycemic conditions. Thiamine has been shown to inhibit this effect of glucose on endothelial cells.
Vitamin B Complex
A group of water-soluble vitamins, some of which are COENZYMES. (See all compounds classified as Vitamin B Complex.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11D - Vitamin b1, plain and in combination with vitamin b6 and b12
A11DA - Vitamin b1, plain
A11DA01 - Thiamine (vit B1)
Absorption
Absorbed mainly from duodenum, by both active and passive processes
Absorption of thiamin occurs mainly in the jejunum. At low concentrations of thiamin, absorption occurs by an active transport system that involves phosphyrylation; at higher concentrations, absorption occurs by passive diffusion. Only a small percentage of a high dose of thiamin is absorbed, and elevated serum values result in active urinary excretion of the vitamin.
Otten JJ, Hellwig JP, Meyers LD, eds; Dietary Reference Intakes: The Essential Guide to Nutrient Requirements, Washington, DC: The National Academies Press, 2006, p. 281
Thiamin is transported in blood in both erythrocytes and plasma and is excreted in the urine.
Otten JJ, Hellwig JP, Meyers LD, eds; Dietary Reference Intakes: The Essential Guide to Nutrient Requirements, Washington, DC: The National Academies Press, 2006, p.281
Thiamine is absorbed from the small intestine and is phosphorylated in the intestinal mucosa.
Furia, T.E. (ed.). CRC Handbook of Food Additives. 2nd ed. Cleveland: The Chemical Rubber Co., 1972., p. 89
The B vitamins are readily absorbed from the gastrointestinal tract, except in malabsorption syndromes. Thiamine is absorbed mainly in the duodenum.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 2647
For more Absorption, Distribution and Excretion (Complete) data for Vitamin B1 (8 total), please visit the HSDB record page.
Hepatic
Converted in vivo to thiamine diphosphate, a coenzyme in the decarboxylation of alpha-keto acids.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1598
Compound 3-(2'-methyl-4'-amino-5'-pyrimidylmethyl)-4-methylthiazole-5-acetic acid, ie thiamine acetic acid, 2-methyl-4-amino-5-formylaminomethylpyrimidine, and 5-(2-hydroxyethyl)-4-methylthiazole have been identified as important metabolites of thiamine, vitamin B1.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 248
Biotransformation of thiamine in mammals is generally supposed to /yield/ thiochrome, thiamine disulfide, 5-(2-hydroxyethyl)-4-methyl-thiazole, and some form corresponding to pyrimidine residue of thiamine.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 229
Thiamine is metabolized in the liver of animals. Several urinary metabolites of thiamine have been identified in humans. Little or no unchanged thiamine is excreted in urine following administration of physiologic doses; however, following administration of larger doses, both unchanged thiamine and metabolites are excreted after tissue stores become saturated.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009)
The biological half-life of the vitamin is in the range of 9-18 days.
Otten JJ, Hellwig JP, Meyers LD, eds; Dietary Reference Intakes: The Essential Guide to Nutrient Requirements, Washington, DC: The National Academies Press, 2006, p.281
With higher pharmacological levels, namely repetitive 250-mg amounts taken orally and 500 mg given intramuscularly, nearly 1 week was required for steady state plasma concentrations to be reached; a mean elimination half-life of 1.8 days was estimated.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press, Washington, D.C., pg. 59, 1998. Available from, as of March 2, 2010: https://www.nap.edu/catalog/6015.html
Total thiamin content of the adult human has been estimated to be approximately 30 mg, and the biological half-life of the vitamin is probably in the range of 9 to 18 days.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press, Washington, D.C., pg. 59, 1998. Available from, as of March 2, 2010: https://www.nap.edu/catalog/6015.html
It is thought that the mechanism of action of thiamine on endothelial cells is related to a reduction in intracellular protein glycation by redirecting the glycolytic flux. Thiamine is mainly the transport form of the vitamin, while the active forms are phosphorylated thiamine derivatives. Natural derivatives of thiamine phosphate, such as thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), also sometimes called thiamine pyrophosphate (TPP), thiamine triphosphate (ThTP), and thiamine triphosphate (AThTP), that act as coenzymes in addition to their each unique biological functions.
Metabolic control analysis predicts that stimulators of transketolase enzyme synthesis such as thiamin (vitamin B-1) support a high rate of nucleic acid ribose synthesis necessary for tumor cell survival, chemotherapy resistance, and proliferation. Metabolic control analysis also predicts that transketolase inhibitor drugs will have the opposite effect on tumor cells. This may have important implications in the nutrition and future treatment of patients with cancer.
PMID:10890024 Cascante M et al; Nutr Cancer 36 (2): 150-4 (2000).

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
18
PharmaCompass offers a list of Vitamin B1 API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vitamin B1 manufacturer or Vitamin B1 supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vitamin B1 manufacturer or Vitamin B1 supplier.
PharmaCompass also assists you with knowing the Vitamin B1 API Price utilized in the formulation of products. Vitamin B1 API Price is not always fixed or binding as the Vitamin B1 Price is obtained through a variety of data sources. The Vitamin B1 Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vitamin B1 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vitamin B1, including repackagers and relabelers. The FDA regulates Vitamin B1 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vitamin B1 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vitamin B1 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vitamin B1 supplier is an individual or a company that provides Vitamin B1 active pharmaceutical ingredient (API) or Vitamin B1 finished formulations upon request. The Vitamin B1 suppliers may include Vitamin B1 API manufacturers, exporters, distributors and traders.
click here to find a list of Vitamin B1 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Vitamin B1 Drug Master File in Japan (Vitamin B1 JDMF) empowers Vitamin B1 API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Vitamin B1 JDMF during the approval evaluation for pharmaceutical products. At the time of Vitamin B1 JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Vitamin B1 suppliers with JDMF on PharmaCompass.
Vitamin B1 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vitamin B1 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vitamin B1 GMP manufacturer or Vitamin B1 GMP API supplier for your needs.
A Vitamin B1 CoA (Certificate of Analysis) is a formal document that attests to Vitamin B1's compliance with Vitamin B1 specifications and serves as a tool for batch-level quality control.
Vitamin B1 CoA mostly includes findings from lab analyses of a specific batch. For each Vitamin B1 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vitamin B1 may be tested according to a variety of international standards, such as European Pharmacopoeia (Vitamin B1 EP), Vitamin B1 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vitamin B1 USP).
