
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
FDF
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
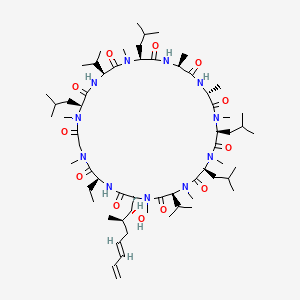

1. Isa 247
2. Isa(tx)247
3. Isa-247
4. Isatx247
5. Lupkynis
1. 515814-01-4
2. Lupkynis
3. Luveniq
4. Isatx-247
5. Voclosporin [usan]
6. Isatx247
7. Isa-247
8. Isa247
9. Lx211
10. Lx-211
11. R 1524
12. R-1524
13. Isa(tx)247
14. (3s,6s,9s,12r,15s,18s,21s,24s,30s,33s)-30-ethyl-33-[(1r,2r,4e)-1-hydroxy-2-methylhepta-4,6-dienyl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone
15. 515814-00-3
16. 2pn063x6b1
17. Trans-isa 247
18. Voclera
19. Isa 247
20. (3s,6s,9s,12r,15s,18s,21s,24s,30s,33s)-30-ethyl-33-((1r,2r,e)-1-hydroxy-2-methylhepta-4,6-dien-1-yl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone
21. Trans-isa-247
22. Voclosporin (usan/inn)
23. Voclosporin [usan:inn]
24. Isatx 247
25. Unii-2pn063x6b1
26. 3odi
27. Lx-214
28. Isatx-247; Luveniq
29. Voclosporin [mi]
30. Voclosporin [inn]
31. Voclosporin [mart.]
32. Voclosporin [who-dd]
33. E-isa247
34. Schembl12632344
35. Gtpl11388
36. Voclosporin [orange Book]
37. Chebi:135957
38. Dtxsid401030488
39. (e)-isa-247
40. Ex-a5922
41. At27977
42. Db11693
43. Hy-106638
44. Cs-0026210
45. R1524
46. D09033
47. Q7939256
48. Cyclosporin A, 6-((2s,3r,4r)-3-hydroxy-4-methyl-2-(methylamino)-6,8-nonadienoic Acid)-
49. (3s,6s,9s,12r,15s,18s,21s,24s,30s,33s)-30-ethyl-33-[(1r,2r,4e)-1-hydroxy-2-methylhepta-4,6-dien-1-yl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-bis(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecone
50. 1,11-anhydro[l-alanyl-d-alanyl-n-methyl-l-leucyl-n-methyl-l-leucyl- N-methyl-l-valyl-[(2s,3r,4r,6e)-3-hydroxy-4-methyl- 2-(methylamino)nona-6,8-dienoyl][(2s)-2-aminobutanoyl]- N-methylglycyl-n-methyl-l-leucyl-l-valyl-n-methyl-l-leucine]
51. Cyclo (((e,z)-(2s,3r,4r)-3-hydroxy-4-methyl-2-(methylamino)nona-6,8-dienoyl)-l-2-aminobytyrl-n-methyl-glycyl-n-methyl-l-leucyl-l-valyl-n-methyl-l-leucyl-l-alanyl-d-alanyl-n-methyl-l-leucyl-n-methyl-l-leucyl-n-methyl-l-valyl)
52. Cyclo(l-alanyl-d-alanyl-n-methyl-l-leucyl-n-methyl-l-leucyl-n-methyl-l-valyl- ((2s,3r,4r,6e)-3-hydroxy-4-methyl-2-(methylamino)nona-6,8-dienoyl)-(2s)-2- Aminobutanoyl-n-methylglycyl-n-methyl-l-leucyl-l-valyl-n-methyl-l-leucyl)
53. Cyclosporin A, 6-((2s,3r,4r,6e)-3-hydroxy-4-methyl-2-(methylamino)-6,8- Nonadienoic Acid)-
| Molecular Weight | 1214.6 g/mol |
|---|---|
| Molecular Formula | C63H111N11O12 |
| XLogP3 | 7.9 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 16 |
| Exact Mass | 1213.84136802 g/mol |
| Monoisotopic Mass | 1213.84136802 g/mol |
| Topological Polar Surface Area | 279 Ų |
| Heavy Atom Count | 86 |
| Formal Charge | 0 |
| Complexity | 2380 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Voclosporin is used in combination with a background immunosuppressive regimen for the treatment of lupus nephritis. Safety has not been established in combination with cyclophosphamide.
Treatment of Systemic Lupus Erythematosus (SLE)
Treatment of non-infectious uveitis
Lupkynis is indicated in combination with mycophenolate mofetil for the treatment of adult patients with active class III, IV or V (including mixed class III/V and IV/V) lupus nephritis (LN).
Voclosporin inhibits calcineurin, leading to the inhibition of T cell activation by blocking the transcription of early inflammatory cytokines. This reduces inflammation in the kidney, treating lupus nephritis and preventing permanent renal damage.
L04AD03
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AD - Calcineurin inhibitors
L04AD03 - Voclosporin
Absorption
When administered on an empty stomach, the median Tmax of voclosporin is 1.5 hours, but can range from 1-4 hours. The AUC is estimated at 7693.6 ng/mL*h and the Cmax is estimated at 955.5 ng/mL.
Route of Elimination
Voclosporin is eliminated in the urine and feces, with about 88% detected in the feces and about 2% detected in the urine.
Volume of Distribution
The apparent volume of distribution of voclosporin is 2,154 L. Voclosporin distributes extensively into red blood cells; distribution between whole blood and plasma is dependent on concentration and temperature.
Clearance
The mean apparent steady-state clearance of voclosporin is 63.6 L/h. Hepatic and renal impairment significantly reduce the clearance of voclosporin.
Voclosporin is mainly metabolized by the CYP3A4 hepatic cytochrome enzyme. Pharmacologic activity is mainly attributed to the parent molecule. A major metabolite has been detected in human whole blood, representing 16.7% of total exposure; this metabolite is about 8-fold less potent than the parent drug, voclosporin.
The average terminal half-life of voclosporin is about 30 hours (24.9 to 36.5 hours).
Through the inhibition of calcineurin, voclosporin blocks IL-2 expression and T-cell mediated immune responses, stabilizing podocytes in the kidneys. Voclospoprin is a cyclosporine A analog. It is structurally similar to cyclosporine A (CsA) with the exception of an amino acid modification in one region. This modification changes the binding of voclosporin to calcineurin. Cyclosporine inhibitors reversibly inhibit T-lymphocytes. They also inhibit lymphokine production and release. Cyclosporine A exerts its inhibitory effects on T-lymphocytes by binding to cyclophilin. A cyclophilin-cyclosporine complex is formed, leading to the inhibition of calcium- and calmodulin-dependent serine-threonine phosphatase activity of calcineurin. Along with calcineurin inhibition, the inhibition of many transcription factors necessary for the induction of various cytokine genes such as IL-2, IFN-, IL-4 and GM-CSF occurs. This, in turn, reduces inflammation, treating renal glomerulonephritis associated with systemic lupus erythematosus.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 Medichem is a vertically integrated pharmaceutical company specializing in the development & manufacturing of APIs & FDFs.
Medichem is a vertically integrated pharmaceutical company specializing in the development & manufacturing of APIs & FDFs.

NDC Package Code : 53296-0130
Start Marketing Date : 2024-08-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (75kg/75kg)
Marketing Category : BULK INGREDIENT
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33779
Submission : 2020-04-06
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2024-08-09
Pay. Date : 2024-06-10
DMF Number : 40089
Submission : 2024-07-24
Status : Active
Type : II

NDC Package Code : 47848-059
Start Marketing Date : 2022-04-04
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (100kg/100kg)
Marketing Category : BULK INGREDIENT

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others

GDUFA
DMF Review : Reviewed
Rev. Date : 2024-09-11
Pay. Date : 2024-07-19
DMF Number : 40162
Submission : 2024-08-01
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF

GDUFA
DMF Review : Reviewed
Rev. Date : 2024-09-19
Pay. Date : 2024-07-11
DMF Number : 39556
Submission : 2024-07-09
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is an approved oral calcineurin inhibitor, a small molecule drug candidate, which is indicated for the treatment of patients with Lupus Nephritis.
Lead Product(s): Voclosporin
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 24, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Aurinia Announces Japan Approval of LUPKYNIS® (Voclosporin) to Treat Lupus Nephritis
Details : Lupkynis (voclosporin) is an approved oral calcineurin inhibitor, a small molecule drug candidate, which is indicated for the treatment of patients with Lupus Nephritis.
Brand Name : Lupkynis
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 24, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a USFDA approved oral calcineurin inhibitor, small molecule drug candidate, which is indicated for the treatment of patients with Lupus Nephritis.
Lead Product(s): Voclosporin
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 30, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
FDA Approves Updated LUPKYNIS® Label with Long-Term Data from AURORA Clinical Program
Details : Lupkynis (voclosporin) is a USFDA approved oral calcineurin inhibitor, small molecule drug candidate, which is indicated for the treatment of patients with Lupus Nephritis.
Brand Name : Lupkynis
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 30, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with a dual mechanism of action. It is being developed for the treatment of lupus nephritis.
Lead Product(s): Voclosporin
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with a dual mechanism of action. It is being developed for the treatment of lupus nephritis.
Brand Name : Lupkynis
Molecule Type : Peptide
Upfront Cash : Not Applicable
November 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is an an oral calcineurin inhibitor which is being evaluated in clinical trials for the treatment of patients with Lupus Nephritis.
Lead Product(s): Voclosporin
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Otsuka Pharmaceutical
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Otsuka Pharmaceutical
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Lupkynis (voclosporin) is an an oral calcineurin inhibitor which is being evaluated in clinical trials for the treatment of patients with Lupus Nephritis.
Brand Name : Lupkynis
Molecule Type : Small molecule
Upfront Cash : Not Applicable
November 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a calcineurin-inhibitor of calcineurin. The immunosuppressant activity results in inhibition of lymphocyte proliferation, T-cell cytokine production, and expression of T-cell activation surface antigens. Currently being developed for lupus nephritis.
Lead Product(s): Voclosporin
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 03, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Aurinia Pharmaceuticals Announces NICE Recommendation of LUPKYNIS® (Voclosporin) For Adults with ...
Details : Lupkynis (voclosporin) is a calcineurin-inhibitor of calcineurin. The immunosuppressant activity results in inhibition of lymphocyte proliferation, T-cell cytokine production, and expression of T-cell activation surface antigens. Currently being develope...
Brand Name : Lupkynis
Molecule Type : Peptide
Upfront Cash : Not Applicable
May 03, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with dual mechanism of action, has received swissmedic approval to treat adults with active class III, IV and V lupus nephritis.
Lead Product(s): Voclosporin
Therapeutic Area: Immunology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Immunology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Aurinia Pharmaceuticals Announces Swissmedic Approval of LUPKYNIS® (voclosporin)
Details : Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with dual mechanism of action, has received swissmedic approval to treat adults with active class III, IV and V lupus nephritis.
Brand Name : Lupkynis
Molecule Type : Peptide
Upfront Cash : Not Applicable
May 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a calcineurin-inhibitor of calcineurin. The immunosuppressant activity results in inhibition of lymphocyte proliferation, T-cell cytokine production, and expression of T-cell activation surface antigens. Currently being developed for lupus nephritis.
Lead Product(s): Voclosporin,Mycophenolate Mofetil,Undisclosed
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: PreclinicalProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin,Mycophenolate Mofetil,Undisclosed
Therapeutic Area : Nephrology
Highest Development Status : Preclinical
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Aurinia Pharmaceuticals Announces New and Refined Method of Use Patent for LUPKYNIS® in the Treat...
Details : Lupkynis (voclosporin) is a calcineurin-inhibitor of calcineurin. The immunosuppressant activity results in inhibition of lymphocyte proliferation, T-cell cytokine production, and expression of T-cell activation surface antigens. Currently being develope...
Brand Name : Lupkynis
Molecule Type : Peptide
Upfront Cash : Not Applicable
April 11, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with a dual mechanism of action, acting as an immunosuppressant through inhibition of T-cell activation and cytokine production and promoting podocyte stability in the kidney.
Lead Product(s): Voclosporin
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 05, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with a dual mechanism of action, acting as an immunosuppressant through inhibition of T-cell activation and cytokine production and promoting podocyte stability in the k...
Brand Name : Lupkynis
Molecule Type : Peptide
Upfront Cash : Not Applicable
April 05, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a calcineurin-inhibitor of calcineurin. The immunosuppressant activity results in inhibition of lymphocyte proliferation, T-cell cytokine production, and expression of T-cell activation surface antigens. Currently being developed for lupus nephritis.
Lead Product(s): Voclosporin,Mycophenolate Mofetil,Undisclosed
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: PreclinicalProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin,Mycophenolate Mofetil,Undisclosed
Therapeutic Area : Nephrology
Highest Development Status : Preclinical
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Lupkynis (voclosporin) is a calcineurin-inhibitor of calcineurin. The immunosuppressant activity results in inhibition of lymphocyte proliferation, T-cell cytokine production, and expression of T-cell activation surface antigens. Currently being develope...
Brand Name : Lupkynis
Molecule Type : Peptide
Upfront Cash : Not Applicable
January 24, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with a dual mechanism of action, acting as an immunosuppressant through inhibition of T-cell activation and cytokine production and promoting podocyte stability in the kidney.
Lead Product(s): Voclosporin
Therapeutic Area: Nephrology Brand Name: Lupkynis
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 30, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voclosporin
Therapeutic Area : Nephrology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Aurinia Announces the Great Britain Marketing Authorization of LUPKYNIS® (voclosporin) for the Tr...
Details : Lupkynis (voclosporin) is a novel, structurally modified calcineurin inhibitor (CNI) with a dual mechanism of action, acting as an immunosuppressant through inhibition of T-cell activation and cytokine production and promoting podocyte stability in the k...
Brand Name : Lupkynis
Molecule Type : Peptide
Upfront Cash : Not Applicable
November 30, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : LUPKYNIS
Dosage Form : CAPSULE;ORAL
Dosage Strength : 7.9MG
Approval Date : 2021-01-22
Application Number : 213716
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2026-01-22
Application Number : 213716
Product Number : 1
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?