



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
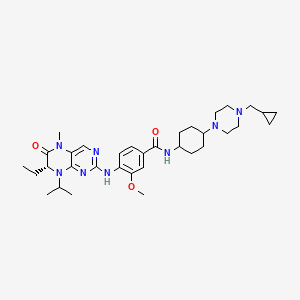

1. Bi 6727
2. Bi-6727
1. 755038-65-4
2. Bi 6727
3. Volasertib (bi 6727)
4. Bi-6727
5. Bi6727 (volasertib)
6. Volasertib [usan]
7. 6em57086ea
8. Volasertib (usan)
9. Bi6727
10. Bi6727(volasertib)
11. Benzamide, N-(trans-4-(4-(cyclopropylmethyl)-1-piperazinyl)cyclohexyl)-4-(((7r)-7-ethyl-5,6,7,8-tetrahydro-5-methyl-8-(1-methylethyl)-6-oxo-2-pteridinyl)amino)-3-methoxy-
12. N-((1s,4s)-4-(4-(cyclopropylmethyl)piperazin-1-yl)cyclohexyl)-4-(((r)-7-ethyl-8-isopropyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl)amino)-3-methoxybenzamide
13. N-(trans-4-(4-(cyclopropylmethyl)piperazin-1-yl)cyclohexyl)-4-(((r)-7-ethyl-8-isopropyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl)amino)-3-methoxybenzamide
14. N-{trans-4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohexyl}-4-{[(7r)-7-ethyl-5-methyl-8-(1-methylethyl)-6-oxo-5,6,7,8-tetrahydropteridin-2-yl]amino}-3-methoxybenzamide
15. Volasertib [usan:inn]
16. Unii-6em57086ea
17. Ibi
18. N-(trans-4-(4-(cyclopropylmethyl)piperazin-1-yl)cyclohexyl)-4-(((7r)-7-ethyl-5-methyl-8-(1-methylethyl)-6-oxo-5,6,7,8-tetrahydropteridin-2-yl)amino)-3-methoxybenzamide
19. Bi6727,volasertib
20. Volasertib(bi6727)
21. Volasertib [inn]
22. Bi6727 - Volasertib
23. Volasertib [who-dd]
24. Mls006011195
25. Schembl738946
26. Gtpl7947
27. Schembl2169101
28. Schembl9888052
29. Chembl1233528
30. Chembl4284151
31. Schembl21916558
32. Bcpp000341
33. Dtxsid801099395
34. Dtxsid901025694
35. Bcp02405
36. Ex-a1364
37. Bdbm50402023
38. Mfcd20926414
39. Nsc757149
40. Nsc800965
41. S2235
42. Zinc39716290
43. Akos030257530
44. Zinc100071772
45. Zinc248087828
46. Bcp9000403
47. Ccg-264952
48. Cs-0274
49. Db12062
50. Nsc-757149
51. Nsc-800965
52. Ncgc00263087-01
53. Ncgc00263087-02
54. Ncgc00263087-03
55. Ncgc00263087-12
56. Ncgc00263087-15
57. Ncgc00485964-01
58. Ac-32844
59. As-16996
60. Hy-12137
61. N-[4-[4-(cyclopropylmethyl)piperazin-1-yl]cyclohexyl]-4-[[(7r)-7-ethyl-5-methyl-6-oxo-8-propan-2-yl-7h-pteridin-2-yl]amino]-3-methoxybenzamide
62. Smr004702964
63. Sw218140-2
64. D10182
65. Sr-03000003275
66. Q7939986
67. Sr-03000003275-1
68. 755038-54-1
69. N-((trans)-4-(4-(cyclopropylmethyl)piperazin-1-yl)cyclohexyl)-4-((r)-7-ethyl-8-isopropyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-ylamino)-3-methoxybenzamide
70. N-[cis-4-[4-(cyclopropylmethyl)-1-piperazinyl]cyclohexyl]-4-[[(7r)-7-ethyl-5,6,7,8-tetrahydro-5-methyl-8-(1-methylethyl)-6-oxo-2-pteridinyl]amino]-3-methoxybenzamide
71. N-[trans-4-[4-(cyclopropylmethyl)-1-piperazinyl]cyclohexyl]-4-[[(7r)-7-ethyl-5,6,7,8-tetrahydro-5-methyl-8-(1-methylethyl)-6-oxo-2-pteridinyl]amino]-3-methoxybenzamide
| Molecular Weight | 618.8 g/mol |
|---|---|
| Molecular Formula | C34H50N8O3 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Exact Mass | 618.40058749 g/mol |
| Monoisotopic Mass | 618.40058749 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 996 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of acute myeloid leukaemia


