

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
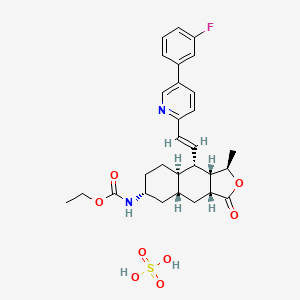

1. Sch 530348
2. Sch-530348
3. Sch530348
4. Vorapaxar
5. Zontivity
1. 705260-08-8
2. Zontivity
3. Sch 530348
4. Vorapaxar Sulfate [usan]
5. Vorapaxar Monosulfate
6. Chebi:83314
7. In66038e6c
8. Vorapaxar (sulfate)
9. Ethyl N-[(1r,3ar,4ar,6r,8ar,9s,9as)-9-[(e)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethenyl]-1-methyl-3-oxo-3a,4,4a,5,6,7,8,8a,9,9a-decahydro-1h-benzo[f][2]benzofuran-6-yl]carbamate;sulfuric Acid
10. Unii-in66038e6c
11. Zontivity (tn)
12. Carbamic Acid, N-((1r,3ar,4ar,6r,8ar,9s,9as)-9-((1e)-2-(5-(3-fluorophenyl)-2-pyridinyl)ethenyl)dodecahydro-1-methyl-3-oxonaphtho(2,3-c)furan-6-yl)-, Ethyl Ester, Sulfate (1:1)
13. Carbamic Acid, N-[(1r,3ar,4ar,6r,8ar,9s,9as)-9-[(1e)-2-[5-(3-fluorophenyl)-2-pyridinyl]ethenyl]dodecahydro-1-methyl-3-oxonaphtho[2,3-c]furan-6-yl]-, Ethyl Ester, Sulfate (1:1)
14. Sch 530348 Sulfate
15. Vorapaxar Sulfate [mi]
16. Vorapaxar Sulfate (jan/usan)
17. Vorapaxar Sulfate [jan]
18. Chembl2107386
19. Dtxsid60990731
20. Vorapaxar Sulfate [vandf]
21. Sch 530348 H2so4 Salt
22. Vorapaxar Sulfate [who-dd]
23. Amy32612
24. Ex-a1149
25. Hy-10119a
26. Mfcd16038877
27. Akos030524402
28. Vorapaxar Sulfate [orange Book]
29. As-35235
30. Ethyl ((1r,3ar,4ar,6r,8ar,9s,9as)-9-((e)-2-(5-(3-fluorophenyl)pyridin-2-yl)vinyl)-1-methyl-3-oxododecahydronaphtho[2,3-c]furan-6-yl)carbamate Sulfate
31. Cs-0002462
32. D09766
33. Sch 530348 Sulfate; Vorapaxar Sulfate; Mk5348
34. Q27156749
35. 2-[(e)-2-{(3r,3as,4s,4ar,7r,8ar,9ar)-7-[(ethoxycarbonyl)amino]-3-methyl-1-oxododecahydronaphtho[2,3-c]furan-4-yl}ethenyl]-5-(3-fluorophenyl)pyridinium Hydrogen Sulfate
36. Carbamic Acid, ((1r,3ar,4ar,6r,8ar,9s,9as)-9-((1e)-2-(5-(3-fluorophenyl)-2-pyridinyl)ethenyl)dodecahydro-1-methyl-3-oxonaphtho(2,3-c)furan-6-yl)-, Ethyl Ester, Sulfate
37. Carbamic Acid, ((1r,3ar,4ar,6r,8ar,9s,9as)-9-((1e)-2-(5-(3-fluorophenyl)-2-pyridinyl)ethenyl)dodecahydro-1-methyl-3-oxonaphtho(2,3-c)furan-6-yl)-, Ethyl Ester, Sulfate (1:1)
38. Ethyl ((1r,3ar,4ar,6r,8ar,9s,9as)-9-((1e)-2-(5-(3-fluorophenyl)pyridin-2-yl)ethenyl)-1-methyl-3-oxododecahydronaphtho(2,3-c)furan-6-yl)carbamate Sulfate
39. Ethyl [(1r,3ar,4ar,6r,8ar,9s,9as)-9-{(e)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethenyl}-1-methyl-3-oxododecahydronaphtho[2,3-c]furan-6-yl]carbamate Sulfate
| Molecular Weight | 590.7 g/mol |
|---|---|
| Molecular Formula | C29H35FN2O8S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 590.20981541 g/mol |
| Monoisotopic Mass | 590.20981541 g/mol |
| Topological Polar Surface Area | 161 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 902 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Zontivityis indicated for the reduction of atherothrombotic events in adult patients with
- a history of myocardial infarction (MI), ,co-administered with acetylsalicylic acid (ASA) and, where appropriate, clopidogrel; or
- symptomatic peripheral arterial disease
(PAD), co-administered with acetylsalicylic acid (ASA) or, where appropriate, clopidogrel.
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
B01
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
