
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Uk 109,496
2. Uk 109496
3. Uk-109,496
4. Uk-109496
5. Uk109,496
6. Uk109496
7. Vfend
1. 137234-62-9
2. Vfend
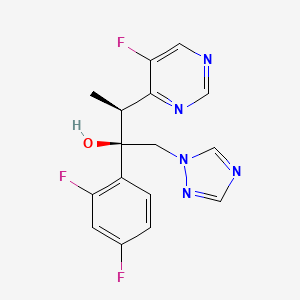
3. (2r,3s)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1h-1,2,4-triazol-1-yl)butan-2-ol
4. Uk-109496
5. (+/-)-voriconazole
6. Voriconazole Vfend
7. Uk-109,496
8. Uk 109496
9. Voriconzole
10. (2r,3s)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1,2,4-triazol-1-yl)butan-2-ol
11. Vcz
12. Voriconazole, (+/-)-
13. Chembl638
14. Nsc-759888
15. Jfu09i87tr
16. Usg4b1cd29
17. Cpd000466350
18. (r-(r*,s*))-alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1h-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol
19. 4-pyrimidineethanol, Alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1h-1,2,4-triazol-1-ylmethyl)-, (alphar,betas)-
20. Chebi:10023
21. 173967-54-9
22. Vrc
23. Voriconazol
24. Dsstox_cid_26485
25. Dsstox_rid_81656
26. Dsstox_gsid_46485
27. (2r,3s)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-((1h)-1,2,4-triazol-1-yl)-butan-2-ol
28. (alphar,betas)-alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha(1h-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol
29. 4-pyrimidineethanol, Alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1h-1,2,4-triazol-1-ylmethyl)-, (r-(r*,s*))-
30. Voriconazole Related Compound A
31. 188416-29-7
32. Voriconazolum
33. Vorikonazole
34. Voriconazole [usan:inn:ban]
35. Drg-0301
36. Smr000466350
37. Cas-137234-62-9
38. Vfend (tn)
39. Vfend I.v.
40. Unii-jfu09i87tr
41. Pfizer
42. Ncgc00164622-01
43. Voriconazole Solution
44. Voriconazole- Bio-x
45. Voriconazole - Vfend
46. (alphar,betas)-alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1h-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol
47. Voriconazole In Combination With Mgcd290
48. Voriconazole [mi]
49. Voriconazole [inn]
50. Voriconazole [jan]
51. Unii-usg4b1cd29
52. Voriconazole [usan]
53. Voriconazole [vandf]
54. Schembl36233
55. Voriconazole [mart.]
56. Mls000759464
57. Mls001424082
58. Mls006010028
59. Voriconazole [usp-rs]
60. Voriconazole [who-dd]
61. Voriconazole [ema Epar]
62. Dtxsid5046485
63. Voriconazole (jp17/usp/inn)
64. Zinc14864
65. Amy8903
66. Voriconazole, >=98% (hplc)
67. Bcpp000019
68. Dtxsid201019420
69. Hms2051n09
70. Hms3260m22
71. Hms3713f12
72. Pharmakon1600-01502346
73. Voriconazole [orange Book]
74. Voriconazole [ep Monograph]
75. Tox21_112241
76. Tox21_500150
77. Voriconazole [usp Monograph]
78. Voriconazole 2.0 Mg/ml In Methanol
79. Ac-823
80. Bdbm50333117
81. Nsc759888
82. S1442
83. Akos005145705
84. Tox21_112241_1
85. Ccg-100941
86. Cs-1227
87. Db00582
88. Hy-w337569
89. Ks-1157
90. Nc00191
91. Nsc 759888
92. Ncgc00164622-02
93. Ncgc00164622-04
94. Ncgc00164622-06
95. Ncgc00260835-01
96. Bv164532
97. Hy-76200
98. ( Inverted Exclamation Marka)-voriconazole
99. Voriconazole Related Compound A Rs [usp]
100. Cs-0448910
101. Sw197571-2
102. V0116
103. C07622
104. D00578
105. Ab00639948-04
106. Ab00639948-06
107. Ab00639948_07
108. Ab00639948_08
109. Voriconazole Related Compound A [usp-rs]
110. Voriconazole, Vetranal(tm), Analytical Standard
111. 234v629
112. A807215
113. Q412236
114. J-006986
115. Voriconazole Related Compound A [usp Impurity]
116. Z2616414875
117. Voriconazole, European Pharmacopoeia (ep) Reference Standard
118. Voriconazole, United States Pharmacopeia (usp) Reference Standard
119. (2r,3s)-2,3-bis(2,4-difluorophenyl)-1-(1h-1,2,4-triazol-1-yl)butan-2-ol
120. 2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1h-1,2,4-triazol-1-yl) Butan-2-ol
121. 4-[[4-[2-(5-ethyl-2-pyridyl)ethoxy]phenyl]methylene]-1-oxo-1,3-thiazolidin-5-one
122. Voriconazole, Pharmaceutical Secondary Standard: Certified Reference Material
123. (.alpha.r,.beta.s)-a-(2,4-difluorophenyl)-5-fluoro-.beta.-methyl-.alpha.-(1h-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol
124. (2r,3s)-2-(2,4-difluoro-phenyl)-3-(5-fluoro-pyrimidin-4-yl)-1-[1,2,4]triazol-1-yl-butan-2-ol
125. (2r,3s)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1h-1,2,4-triazol-1-yl) Butan-2-ol
126. (2r,3s)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1h-1,2,4-triazol-1-yl)-2-butanol
127. (2r,3s)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1h-1,2,4-triazol-1-yl)butan-2-ol
128. 2r,3s-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1h-1,2,4-triazol-1-yl)butan-2-ol-4-pyrimidineethanol, ?-(2,4-difluorophenyl)-5-fluoro-?-methyl-?-(1h-1,2,4-triazol-1-ylmethyl)-,?(?r,?s)-
129. 4-pyrimidineethanol, .alpha.-(2,4-difluorophenyl)-5-fluoro-.beta.-methyl-.alpha.-(1h-1,2,4-triazol-1-ylmethyl)-, (.alpha.r,.beta.s)-rel-
130. 4-pyrimidineethanol, A-(2,4-difluorophenyl)-5-fluoro-.beta.-methyl-.alpha.-(1h-1,2,4-triazol-1-ylmethyl)-, (.alpha.r,.beta.s)-
131. 4-pyrimidineethanol, Alpha-(2,4-difluorophenyl)-5-fluoro-beta-methyl-alpha-(1h-1,2,4-triazol-1-ylmethyl)-, (alphar,betas)-rel-
| Molecular Weight | 349.31 g/mol |
|---|---|
| Molecular Formula | C16H14F3N5O |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 349.11504457 g/mol |
| Monoisotopic Mass | 349.11504457 g/mol |
| Topological Polar Surface Area | 76.7 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 448 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Vfend |
| PubMed Health | Voriconazole (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | VFEND (voriconazole), a triazole antifungal agent, is available as a lyophilized powder for solution for intravenous infusion, film-coated tablets for oral administration, and as a powder for oral suspension. The structural formula is:Voriconazole... |
| Active Ingredient | Voriconazole |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | injection; oral; Iv (infusion); Oral; iv (infusion) |
| Strength | 200mg/5ml; 200mg; 200mg/vial; 50mg |
| Market Status | Prescription |
| Company | Pfizer |
| 2 of 4 | |
|---|---|
| Drug Name | Voriconazole |
| PubMed Health | Voriconazole |
| Drug Classes | Antifungal |
| Drug Label | VFEND (voriconazole), a triazole antifungal agent, is available as a lyophilized powder for solution for intravenous infusion, film-coated tablets for oral administration, and as a powder for oral suspension. The structural formula is:Voriconazole... |
| Active Ingredient | Voriconazole |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | Iv (infusion); Oral |
| Strength | 200mg/5ml; 200mg; 200mg/vial; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Sandoz; Teva Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Vfend |
| PubMed Health | Voriconazole (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | VFEND (voriconazole), a triazole antifungal agent, is available as a lyophilized powder for solution for intravenous infusion, film-coated tablets for oral administration, and as a powder for oral suspension. The structural formula is:Voriconazole... |
| Active Ingredient | Voriconazole |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | injection; oral; Iv (infusion); Oral; iv (infusion) |
| Strength | 200mg/5ml; 200mg; 200mg/vial; 50mg |
| Market Status | Prescription |
| Company | Pfizer |
| 4 of 4 | |
|---|---|
| Drug Name | Voriconazole |
| PubMed Health | Voriconazole |
| Drug Classes | Antifungal |
| Drug Label | VFEND (voriconazole), a triazole antifungal agent, is available as a lyophilized powder for solution for intravenous infusion, film-coated tablets for oral administration, and as a powder for oral suspension. The structural formula is:Voriconazole... |
| Active Ingredient | Voriconazole |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | Iv (infusion); Oral |
| Strength | 200mg/5ml; 200mg; 200mg/vial; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Sandoz; Teva Pharms |
For the treatment of esophageal candidiasis, cadidemia, invasive pulmonary aspergillosis, and serious fungal infections caused by Scedosporium apiospermum and Fusarium spp.
FDA Label
Voriconazole is a broad spectrum, triazole antifungal agent and is indicated in adults and children aged 2 years and above as follows:
- treatment of invasive aspergillosis;
- treatment of candidaemia in non-neutropenic patients;
- treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei);
- treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp.
Voriconazole should be administered primarily to patients with progressive, possibly life-threatening infections.
Prophylaxis of invasive fungal infections in high risk allogeneic hematopoietic stem cell transplant (HSCT)
recipients.
Voriconazole, is a broad spectrum, triazole antifungal agent and is indicated in adults and children aged 2 years and above as follows:
- treatment of invasive aspergillosis;
- treatment of in candidaemianon-neutropenic patients;
- treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei);
- Treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp.
Vfend should be administered primarily to patients with progressive, possibly life-threatening infections.
Prophylaxis of invasive fungal infections in high risk allogeneic hematopoietic stem cell transplant (HSCT) recipients.
Voriconazole is a broad-spectrum, triazole antifungal agent and is indicated in adults and children aged two years and above as follows:
- treatment of invasive aspergillosis;
- treatment of candidaemia in non-neutropenic patients;
- treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei);
- Treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp.
Voriconazole Accord should be administered primarily to patients with progressive, possibly life-threatening infections.
Treatment of candidaemia in non-neutropenic patients, Treatment of fluconazole-resistant serious invasive Candida infections (including C. krusei), Treatment of invasive aspergillosis, Treatment of serious fungal infections caused by Scedosporium spp. and Fusarium spp.
Voriconazole is a fungistatic triazole antifungal used to treat infections by inhibiting fungal growth. It is known to cause hepatotoxic and photosensitivity reactions in some patients.
14-alpha Demethylase Inhibitors
Compounds that specifically inhibit STEROL 14-DEMETHYLASE. A variety of azole-derived ANTIFUNGAL AGENTS act through this mechanism. (See all compounds classified as 14-alpha Demethylase Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
J02AC03
J02AC03
J02AC03
J02AC03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J02 - Antimycotics for systemic use
J02A - Antimycotics for systemic use
J02AC - Triazole and tetrazole derivatives
J02AC03 - Voriconazole
Absorption
The oral bioavailability is estimated to be 96% in healthy adults. Population pharmacokinetic studies report a reduced bioavailability pediatric patients with a mean of 61.8% (range 44.664.5%) thought to be due to differences in first-pass metabolism or due to differences in diet. Of note, transplant patients also have reduced bioavailability but this is known to increase with time after transplantation and may be due in part to gastrointestinal upset from surgery and some transplant medications. Tmax is 1-2 hours with oral administration. When administered with a high-fat meal Cmax decreases by 34% and AUC by 24%. pH does not have an effect on absorption of voriconazole. Differences in Cmax and AUC have been observed between healthy adult males and females with Cmax increasing by 83% and AUC by 113% although this has not been observed to significantly impact medication safety profiles.
Route of Elimination
Voriconazole is eliminated via hepatic metabolism with less than 2% of the dose excreted unchanged in the urine.
Volume of Distribution
The estimated volume of distribution of voriconazole is 4.6 L/kg. Population pharmacokinetic studies estimate the median volume of distribution to be 77.6 L with the central compartment estimated at 1.07 L/kg Voriconazole is known to achieve therapeutic concentrations in many tissues including the brain, lungs, liver, spleen, kidneys, and heart.
Clearance
The clearance of voriconazole is estimated to be a mean of 5.25-7 L/h in healthy adults for the linear portion of the drug's kinetics.
Voriconazole undergoes extensive hepatic metabolism through cytochrome enzymes CYP2C9, CYP2C19, and CYP3A4. CYP2C19 mediates N-oxidation with an apparent Km of 14 M and an apparent Vmax of 0.22 nmol/min/nmol CYP2C19. Voriconazole N-oxide is the major circulating metabolite, accounting for 72% of radiolabeled metabolites found. CYP3A4 contributes to N-oxidation with a Km of 16 M and Vmax of 0.05 nmol/min/nmol CYP3A4 as well as 4-hydroxylation with a Km of 11 M and a Vmax of 0.10 nmol/min/nmol CYP3A4. CYP3A5 and CYP3A7 provide minor contributions to N-oxidation and 4-hydroxylation. The N-oxide and 4-hydroxylated metabolites undergo glucuronidation and are excreted through the urine with other minor glucuronidated metabolites.
Voriconazole has known human metabolites that include Hydroxymethyl Voriconazole and Voriconazole N-Oxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Voriconazole follows non-linear kinetics and has a terminal half-life of elimination which is dose-dependent.
Voriconazole is used to treat fungal infections caused by a variety of organisms but including _Aspergillus spp._ and _Candida spp_. Voriconazole is a triazole antifungal exhibiting fungistatic activity against fungal pathogens. Like other triazoles, voriconazole binds to 14-alpha sterol demethylase, also known as CYP51, and inhibits the demethylation of lanosterol as part of the ergosterol synthesis pathway in yeast and other fungi. The lack of sufficient ergosterol disrupts fungal cell membrane function and limits fungal cell growth. With fungal growth limited, the host's immune system is able to clear the invading organism.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37261
Submission : 2022-06-30
Status : Active
Type : II

Date of Issue : 2024-02-12
Valid Till : 2027-02-11
Written Confirmation Number : WC-0407
Address of the Firm :

| Available Reg Filing : ASMF, CN, BR |


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38261
Submission : 2023-03-31
Status : Active
Type : II

| Available Reg Filing : ASMF, CN, BR |


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35869
Submission : 2023-01-16
Status : Active
Type : II

Certificate Number : CEP 2023-030 - Rev 00
Issue Date : 2024-07-01
Type : Chemical
Substance Number : 2576
Status : Valid



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37702
Submission : 2022-12-29
Status : Active
Type : II

Certificate Number : CEP 2022-332 - Rev 00
Issue Date : 2024-12-09
Type : Chemical
Substance Number : 2576
Status : Valid

Date of Issue : 2023-08-14
Valid Till : 2026-03-08
Written Confirmation Number : WC-0465
Address of the Firm :



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36448
Submission : 2021-12-24
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
GDUFA
DMF Review : Complete
Rev. Date : 2017-07-11
Pay. Date : 2017-04-28
DMF Number : 31545
Submission : 2017-05-10
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38261
Submission : 2023-03-31
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37261
Submission : 2022-06-30
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2014-01-14
Pay. Date : 2013-12-13
DMF Number : 24717
Submission : 2011-03-31
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25100
Submission : 2011-07-04
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-02-08
Pay. Date : 2012-11-26
DMF Number : 21443
Submission : 2008-03-18
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-09-21
Pay. Date : 2013-09-11
DMF Number : 21523
Submission : 2008-04-08
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-12-04
Pay. Date : 2015-07-14
DMF Number : 25343
Submission : 2011-09-23
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-06-23
Pay. Date : 2013-09-03
DMF Number : 25707
Submission : 2011-12-20
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for pharmaceutical and biotech industries. LGM also operates as a full-service CDMO, offering formulat...
About the Company : HRV Pharma is a global manufacturer, seller, and exporter of APIs, intermediates, pellets, food-grade chemicals, food additives, and food ingredients. The company provides sourcing...
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
About the Company : Pharmathen, established in 1969, has emerged as a leading in-house development partner in Europe. It specializes in the development, registration, manufacturing & life-cycle manage...
About the Company : Zeon Pharma Industries India Pvt. Ltd. is an ISO 9001:2015, cGMP, and WHO-GMP certified company with a dedicated manufacturing facility for Bulk Drugs (APIs), phytochemicals, herba...
About the Company : Shamrock Pharmachemi Pvt Ltd. is a globally recognized API leader with 26+ years in human and veterinary pharmaceuticals. Operating in 40+ countries, it owns two Gujarat facilities...
About the Company : Maithri Drugs Pvt. Ltd. is a global supplier of Active Pharmaceutical Ingredients (APIs), serving pharmaceutical companies in 60+ countries. Its API portfolio spans antivirals, ant...
About the Company : Apicore LLC, a wholly owned subsidiary of RK Pharma Inc is a leading process R&D and API manufacturing service provider for the worldwide pharmaceutical industry. We offer a wide p...

About the Company : Fuan Pharmaceutical (Group) Co., Ltd. was established on February 25, 2004. The company is located in Chongqing Changshou Economic and Technological Development Zone, covering an a...

About the Company : MN Pharmaceuticals is one of the leading and oldest pharmaceutical companies in Turkey. The company was established in 1923 in a district of İstanbul known as Üsküdar, İhsaniye...

About the Company : Zhejiang Huahai Pharmaceuticals Co., Ltd.was initially founded in 1989, and the company's stock was successfully listed in Shanghai Stock Exchange in March, 2003. Huahai Pharmaceu...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Details:
Busulfan is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Leukemia, Myeloid, Acute.
Lead Product(s): Busulfan,Fludarabine Phosphate,Melphalan,Cyclophosphamide,Posaconazole,Voriconazole,Tacrolimus,Mycophenolate Mofetil
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: National Marrow Donor Program | Incyte Corporation
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 05, 2025

Lead Product(s) : Busulfan,Fludarabine Phosphate,Melphalan,Cyclophosphamide,Posaconazole,Voriconazole,Tacrolimus,Mycophenolate Mofetil
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : National Marrow Donor Program | Incyte Corporation
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Busulfan is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Leukemia, Myeloid, Acute.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 05, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Repotrectinib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Lead Product(s): Repotrectinib,Voriconazole,Quinidine
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Repotrectinib,Voriconazole,Quinidine
Therapeutic Area : Undisclosed
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Repotrectinib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
July 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TFF VORI (voriconazole) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for invasive pulmonary aspergillosis & allergic bronchopulmonary aspergillosis.
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: TFF VORI
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
TFF Pharmaceuticals Updates Data from Clinical Programs on Tacrolimus and Voriconazole
Details : TFF VORI (voriconazole) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for invasive pulmonary aspergillosis & allergic bronchopulmonary aspergillosis.
Product Name : TFF VORI
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TFF VORI (voriconazole inhalation powder) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole in development for the treatment and prevention of invasive pulmonary aspergillosis (IPA), a severe fungal pulmonary disease.
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: TFF VORI
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 31, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : TFF VORI (voriconazole inhalation powder) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole in development for the treatment and prevention of invasive pulmonary aspergillosis (IPA), a severe fungal pulmonary disease.
Product Name : TFF VORI
Product Type : Miscellaneous
Upfront Cash : Inapplicable
July 31, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Voriconazole is a Other Small Molecule drug candidate, which is currently being evaluated in clinical studies for the treatment of Invasive Pulmonary Aspergillosis.
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: UndisclosedProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Undisclosed
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Voriconazole Inhalation Powder for the Treatment of Pulmonary Aspergillosis
Details : Voriconazole is a Other Small Molecule drug candidate, which is currently being evaluated in clinical studies for the treatment of Invasive Pulmonary Aspergillosis.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Voriconazole is a triazole antifungal agent. The primary mode of action of voriconazole is the inhibition of fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylation, an essential step in fungal ergosterol biosynthesis.
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Vfend-Generic
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Eugia Pharma Receives USFDA Approval for Voriconazole for Injection, 200 mg/Vial Single-Dose Vial
Details : Voriconazole is a triazole antifungal agent. The primary mode of action of voriconazole is the inhibition of fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylation, an essential step in fungal ergosterol biosynthesis.
Product Name : Vfend-Generic
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 02, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TFF Pharmaceuticals intends to use the net proceeds from the offering for clinical trials, research and development, working capital and general corporate purposes.
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: TFF VORI
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Jonestrading Institutional Services
Deal Size: $12.3 million Upfront Cash: Undisclosed
Deal Type: Public Offering November 29, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Jonestrading Institutional Services
Deal Size : $12.3 million
Deal Type : Public Offering
TFF Pharmaceuticals Announces Closing of $12.3 Million Public Offering
Details : TFF Pharmaceuticals intends to use the net proceeds from the offering for clinical trials, research and development, working capital and general corporate purposes.
Product Name : TFF VORI
Product Type : Miscellaneous
Upfront Cash : Undisclosed
November 29, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TFF VORI (voriconazole) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for the treatment and prevention of Invasive Pulmonary Aspergillosis (IPA).
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: TFF VORI
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 01, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : TFF VORI (voriconazole) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for the treatment and prevention of Invasive Pulmonary Aspergillosis (IPA).
Product Name : TFF VORI
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 01, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TFF VORI (voriconazole) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for the treatment and prevention of Invasive Pulmonary Aspergillosis (IPA).
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: TFF VORI
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 01, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : TFF VORI (voriconazole) is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for the treatment and prevention of Invasive Pulmonary Aspergillosis (IPA).
Product Name : TFF VORI
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 01, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TFF VORI is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for the treatment and prevention of Invasive Pulmonary Aspergillosis (IPA).
Lead Product(s): Voriconazole,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: TFF VORI
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 08, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Voriconazole,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : TFF VORI is a next-generation, direct-to-lung, inhaled dry powder formulation of voriconazole for the treatment and prevention of Invasive Pulmonary Aspergillosis (IPA).
Product Name : TFF VORI
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 08, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : VORICONAZOLE
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : 200MG/5ML
Approval Date : 2016-04-13
Application Number : 205034
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : VORICONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date : 2016-05-24
Application Number : 206747
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : VORICONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date : 2016-09-07
Application Number : 207049
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : VORICONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2016-09-07
Application Number : 207049
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : VORICONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2015-09-02
Application Number : 203503
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : VFEND
Dosage Form : Tablet; Oral
Dosage Strength : 200MG
Approval Date :
Application Number : 21464
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : VORICONAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2016-08-08
Application Number : 206654
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VORICONAZOLE
Dosage Form : POWDER;INTRAVENOUS
Dosage Strength : 200MG/VIAL
Approval Date : 2012-05-30
Application Number : 90862
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VORICONAZOLE
Dosage Form : POWDER;INTRAVENOUS
Dosage Strength : 200MG/VIAL
Approval Date : 2018-11-30
Application Number : 211661
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VORICONAZOLE
Dosage Form : POWDER;INTRAVENOUS
Dosage Strength : 200MG/VIAL
Approval Date : 2023-03-09
Application Number : 211264
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
68
PharmaCompass offers a list of Voriconazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Voriconazole manufacturer or Voriconazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Voriconazole manufacturer or Voriconazole supplier.
PharmaCompass also assists you with knowing the Voriconazole API Price utilized in the formulation of products. Voriconazole API Price is not always fixed or binding as the Voriconazole Price is obtained through a variety of data sources. The Voriconazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Voriconazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Voriconazole, including repackagers and relabelers. The FDA regulates Voriconazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Voriconazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Voriconazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Voriconazole supplier is an individual or a company that provides Voriconazole active pharmaceutical ingredient (API) or Voriconazole finished formulations upon request. The Voriconazole suppliers may include Voriconazole API manufacturers, exporters, distributors and traders.
click here to find a list of Voriconazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Voriconazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Voriconazole active pharmaceutical ingredient (API) in detail. Different forms of Voriconazole DMFs exist exist since differing nations have different regulations, such as Voriconazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Voriconazole DMF submitted to regulatory agencies in the US is known as a USDMF. Voriconazole USDMF includes data on Voriconazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Voriconazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Voriconazole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Voriconazole Drug Master File in Japan (Voriconazole JDMF) empowers Voriconazole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Voriconazole JDMF during the approval evaluation for pharmaceutical products. At the time of Voriconazole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Voriconazole suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Voriconazole Drug Master File in Korea (Voriconazole KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Voriconazole. The MFDS reviews the Voriconazole KDMF as part of the drug registration process and uses the information provided in the Voriconazole KDMF to evaluate the safety and efficacy of the drug.
After submitting a Voriconazole KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Voriconazole API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Voriconazole suppliers with KDMF on PharmaCompass.
A Voriconazole CEP of the European Pharmacopoeia monograph is often referred to as a Voriconazole Certificate of Suitability (COS). The purpose of a Voriconazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Voriconazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Voriconazole to their clients by showing that a Voriconazole CEP has been issued for it. The manufacturer submits a Voriconazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Voriconazole CEP holder for the record. Additionally, the data presented in the Voriconazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Voriconazole DMF.
A Voriconazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Voriconazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Voriconazole suppliers with CEP (COS) on PharmaCompass.
A Voriconazole written confirmation (Voriconazole WC) is an official document issued by a regulatory agency to a Voriconazole manufacturer, verifying that the manufacturing facility of a Voriconazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Voriconazole APIs or Voriconazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Voriconazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Voriconazole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Voriconazole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Voriconazole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Voriconazole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Voriconazole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Voriconazole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Voriconazole suppliers with NDC on PharmaCompass.
Voriconazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Voriconazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Voriconazole GMP manufacturer or Voriconazole GMP API supplier for your needs.
A Voriconazole CoA (Certificate of Analysis) is a formal document that attests to Voriconazole's compliance with Voriconazole specifications and serves as a tool for batch-level quality control.
Voriconazole CoA mostly includes findings from lab analyses of a specific batch. For each Voriconazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Voriconazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Voriconazole EP), Voriconazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Voriconazole USP).

