
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
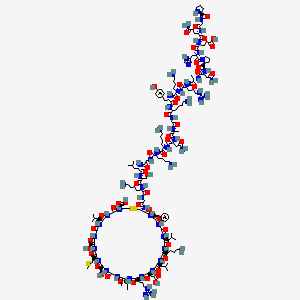

1. Bmn 111
2. Voxzogo
1. Voxzogo
2. Bmn 111
3. Bmn-111
4. Vosoritide [usan:inn]
5. Unii-7se5582q2p
6. 1480724-61-5
7. Gtpl9068
8. 7se5582q2p
| Molecular Weight | 4103 g/mol |
|---|---|
| Molecular Formula | C176H290N56O51S3 |
| XLogP3 | -20.6 |
| Hydrogen Bond Donor Count | 61 |
| Hydrogen Bond Acceptor Count | 64 |
| Rotatable Bond Count | 112 |
| Exact Mass | 4101.1016174 g/mol |
| Monoisotopic Mass | 4100.0982626 g/mol |
| Topological Polar Surface Area | 1820 Ų |
| Heavy Atom Count | 286 |
| Formal Charge | 0 |
| Complexity | 9600 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 32 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vosoritide is indicated for the promotion of linear growth in pediatric patients with achondroplasia who are 5 years of age and older with open epiphyses.
Voxzogo is indicated for the treatment of achondroplasia in patients 2 years of age and older whose epiphyses are not closed. The diagnosis of achondroplasia should be confirmed by appropriate genetic testing.
Vosoritide is an analog of C-type natriuretic peptide that promotes bone growth to combat growth suppression in children with achondroplasia. Urinary cyclic guanosine monophosphate (cGMP) and serum collagen type X marker (CXM) are both elevated following daily therapy with vosoritide and serve as biomarkers for evidence of increased endochondral bone growth, with cGMP indicative of NPR-B binding activity and CXM indicative of bone metabolism. Although relatively well-tolerated, transient episodes of hypotension have been observed in clinical studies. Patients with pre-existing cardiovascular disease and those taking antihypertensive medications were excluded from clinical trials. The risk of hypotension may be reduced by ensuring adequate food and fluid intake prior to the administration of vosoritide. The use of vosoritide in patients with an eGFR <60 mL/min/1.73m2 should also be avoided as there are no data on the influence of renal impairment on its pharmacokinetics.
M05BX
M - Musculo-skeletal system
M05 - Drugs for treatment of bone diseases
M05B - Drugs affecting bone structure and mineralization
M05BX - Other drugs affecting bone structure and mineralization
M05BX07 - Vosoritide
Absorption
In patients receiving daily subcutaneous injections of vosoritide 15 mcg/kg, the mean Cmax ranged from 4.71-7.18 ng/mL and the mean AUC0-t ranged from 161-290 ng-min/mL. The median Tmax following subcutaneous injection was approximately 15 minutes.
Volume of Distribution
The mean apparent volume of distribution following the subcutaneous administration of 15 mcg/kg of vosoritide ranged from 2880 to 3020 mL/kg.
Clearance
The mean apparent clearance following the subcutaneous administration of 15 mcg/kg of vosoritide ranged from 79.4 to 104 mL/min/kg.
As with other therapeutic proteins, vosoritide is likely metabolized via catabolic pathways into smaller peptides and amino acids.
The mean half-life following the subcutaneous administration of 15 mcg/kg of vosoritide ranged from 21.0 to 27.9 minutes.
Achondroplasia is a congenital disease resulting from a missense mutation in the fibroblast growth factor receptor 3 (_FGFR3_) gene, resulting in a gain-of-function that negatively regulates endochondral bone growth. Under normal conditions, _FGFR3_ is expressed during both embryonic and postnatal development, but serves a different role in each. During initial development, FGFR3 signaling promotes proliferation of chondrocytes (i.e. growth), whereas postnatal skeletal growth is actually inhibited by FGFR3 - as a result, the pathologic activation of FGFR3 observed in patients with achondroplasia leads to suppressed pre-pubertal skeletal growth. Vosoritide is an analog of C-type natriuretic peptide (CNP), a signaling molecule that appears primarily responsible for the stimulation of chondrocytes and the growth of long bones. The binding of CNP (or vosoritide) with its corresponding receptor, NPR-B, results in a signaling cascade that ultimately inhibits the MAPK/ERK pathway via inhibition of RAF-1 and stimulates the proliferation and differentiation of chondrocytes. This activity serves to antagonize the downstream signaling resulting from FGFR3 and its resultant effects on bone growth.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
67
PharmaCompass offers a list of Vosoritide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vosoritide manufacturer or Vosoritide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vosoritide manufacturer or Vosoritide supplier.
PharmaCompass also assists you with knowing the Vosoritide API Price utilized in the formulation of products. Vosoritide API Price is not always fixed or binding as the Vosoritide Price is obtained through a variety of data sources. The Vosoritide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vosoritide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vosoritide, including repackagers and relabelers. The FDA regulates Vosoritide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vosoritide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vosoritide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vosoritide supplier is an individual or a company that provides Vosoritide active pharmaceutical ingredient (API) or Vosoritide finished formulations upon request. The Vosoritide suppliers may include Vosoritide API manufacturers, exporters, distributors and traders.
click here to find a list of Vosoritide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Vosoritide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vosoritide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vosoritide GMP manufacturer or Vosoritide GMP API supplier for your needs.
A Vosoritide CoA (Certificate of Analysis) is a formal document that attests to Vosoritide's compliance with Vosoritide specifications and serves as a tool for batch-level quality control.
Vosoritide CoA mostly includes findings from lab analyses of a specific batch. For each Vosoritide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vosoritide may be tested according to a variety of international standards, such as European Pharmacopoeia (Vosoritide EP), Vosoritide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vosoritide USP).
