
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Weekly News Recap #Phispers
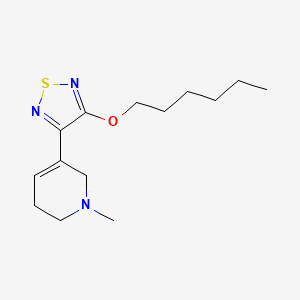

1. (3-o-hexyloxy)-tztp
2. 3-(3-o-hexyl-1,2,5-thiadiazol-4-yl)-1,2,5,6-tetrahydro-1-methylpyridine
3. Ly 246708
4. Ly-246708
5. Ly246708
6. Xanomeline Tartrate
1. 131986-45-3
2. 3-(hexyloxy)-4-(1-methyl-1,2,5,6-tetrahydropyridin-3-yl)-1,2,5-thiadiazole
3. Ly 246708
4. Ly-246708
5. Ly246708
6. 3-hexoxy-4-(1-methyl-3,6-dihydro-2h-pyridin-5-yl)-1,2,5-thiadiazole
7. 9ori6l73cj
8. Chembl21536
9. 5-(4-hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2,3,6-tetrahydro-pyridine
10. Chebi:10056
11. Ly-246708 Free Base
12. 5-[4-(hexyloxy)-1,2,5-thiadiazol-3-yl]-1-methyl-1,2,3,6-tetrahydropyridine
13. Xanomeline (usan)
14. Xanomeline [usan]
15. Pyridine, 3-(4-(hexyloxy)-1,2,5-thiadiazol-3-yl)-1,2,5,6-tetrahydro-1-methyl-
16. Xanomeline [usan:inn]
17. Unii-9ori6l73cj
18. Lumeron
19. Memcor
20. Hexyloxy-tztp
21. Pyridine, 3-[4-(hexyloxy)-1,2,5-thiadiazol-3-yl]-1,2,5,6-tetrahydro-1-methyl-
22. Xanomeline [mi]
23. Xanomeline [inn]
24. Gtpl57
25. 3-(4-(hexyloxy)-1,2,5-thiadiazol-3-yl)-1,2,5,6-tetrahydro-1-methylpyridine
26. Xanomeline [mart.]
27. Xanomeline [who-dd]
28. Schembl121046
29. Dtxsid60157286
30. Bcp31492
31. Ex-a6592
32. Zinc1532358
33. Bdbm50003359
34. Mfcd00867179
35. Akos016007489
36. Db15357
37. Sb18821
38. Nnc-11-0232
39. Ds-15663
40. Hy-105182
41. Cs-0025224
42. Ft-0602214
43. C72296
44. D06330
45. A888347
46. L000694
47. Q8042940
48. Ly-246708; Ly246708; Ly 246708
49. 3-(4-hexoxy-1,2,5-thiadiazol-3-yl)-1-methyl-5,6-dihydro-2h-pyridine
50. 3-(4-hexyloxy-1 ,2,5-thiadiazol-3-yl)-1 ,2,5,6-tetrahydro-1-methyl-pyridine
51. 5-(4-hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2,3,6-tetrahydro-pyridine; Oxalic Acid
52. 5-[4-(hexyloxy)-1,2,5-thiadiazol-3-yl]-1-methyl-1,2,3,6-tetrahydropyridine, Aldrichcpr
53. 1018845-70-9
| Molecular Weight | 281.42 g/mol |
|---|---|
| Molecular Formula | C14H23N3OS |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 281.15618354 g/mol |
| Monoisotopic Mass | 281.15618354 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 298 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Muscarinic Agonists
Drugs that bind to and activate muscarinic cholinergic receptors (RECEPTORS, MUSCARINIC). Muscarinic agonists are most commonly used when it is desirable to increase smooth muscle tone, especially in the GI tract, urinary bladder and the eye. They may also be used to reduce heart rate. (See all compounds classified as Muscarinic Agonists.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)
Psychotropic Drugs
A loosely defined grouping of drugs that have effects on psychological function. Here the psychotropic agents include the antidepressive agents, hallucinogens, and tranquilizing agents (including the antipsychotics and anti-anxiety agents). (See all compounds classified as Psychotropic Drugs.)
 Delivering quality and niche Active Pharmaceutical Ingredients across the global from our USFDA-approved facility.
Delivering quality and niche Active Pharmaceutical Ingredients across the global from our USFDA-approved facility.






 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
NDC Package Code : 58032-3003
Start Marketing Date : 2023-03-17
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
NDC Package Code : 57572-0727
Start Marketing Date : 2024-11-25
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Delivering quality and niche Active Pharmaceutical Ingredients across the global from our USFDA-approved facility.
Delivering quality and niche Active Pharmaceutical Ingredients across the global from our USFDA-approved facility.
About the Company : Mankind Pharma, came into existence in 1986. In 1991, the company was formed into a legal corporation. However, it actively started working as a fully integrated pharmaceutical com...
About the Company : Alfa Chemicals offers an all-encompassing range of solutions to the global pharmaceutical market, including contract manufacturing for intermediates & APIs; supply chain consolidat...

About the Company : BOC Sciences is a brand of BOCSCI Inc. We leverage our wide spectrum of business in the fields of development, manufacturing, marketing, and distribution to help you make best-info...

About the Company : FandaChem, headquartered in Hangzhou, China, is a reputable supplier and exporter of chemicals with a focus on delivering high-quality products. Our expertise lies in exporting a w...

About the Company : Hunan Huateng Pharmaceutical Co., Ltd. is a research-based manufacturer and supplier of combinatorial building blocks, organics and fine chemicals, with 5000m2 laboratory and over ...

About the Company : Raghava Life Sciences Pvt. Ltd. (RLS), established in the year 2018, is an integrated small molecules chemistry Contract research, Development and Manufacturing Organization (CDMO)...

About the Company : "SimSon Pharma, a renowned name in pharmaceutical sector, has build high its reputation as a complete solution provider in contract research arena. We are leading manufacturer and ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

Reply
12 Nov 2024
Reply
29 Oct 2024
Reply
17 Oct 2024

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
ABOUT THIS PAGE
56
PharmaCompass offers a list of Xanomeline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Xanomeline manufacturer or Xanomeline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Xanomeline manufacturer or Xanomeline supplier.
PharmaCompass also assists you with knowing the Xanomeline API Price utilized in the formulation of products. Xanomeline API Price is not always fixed or binding as the Xanomeline Price is obtained through a variety of data sources. The Xanomeline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A xanomeline tartrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of xanomeline tartrate, including repackagers and relabelers. The FDA regulates xanomeline tartrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. xanomeline tartrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of xanomeline tartrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A xanomeline tartrate supplier is an individual or a company that provides xanomeline tartrate active pharmaceutical ingredient (API) or xanomeline tartrate finished formulations upon request. The xanomeline tartrate suppliers may include xanomeline tartrate API manufacturers, exporters, distributors and traders.
click here to find a list of xanomeline tartrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing xanomeline tartrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for xanomeline tartrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture xanomeline tartrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain xanomeline tartrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a xanomeline tartrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of xanomeline tartrate suppliers with NDC on PharmaCompass.
xanomeline tartrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of xanomeline tartrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right xanomeline tartrate GMP manufacturer or xanomeline tartrate GMP API supplier for your needs.
A xanomeline tartrate CoA (Certificate of Analysis) is a formal document that attests to xanomeline tartrate's compliance with xanomeline tartrate specifications and serves as a tool for batch-level quality control.
xanomeline tartrate CoA mostly includes findings from lab analyses of a specific batch. For each xanomeline tartrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
xanomeline tartrate may be tested according to a variety of international standards, such as European Pharmacopoeia (xanomeline tartrate EP), xanomeline tartrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (xanomeline tartrate USP).
