
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
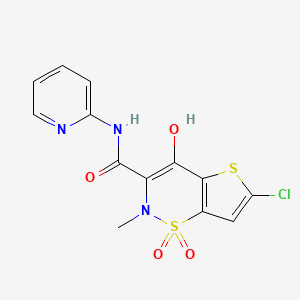

1. Chlortenoxicam
2. Xefocam
1. 70374-39-9
2. Chlortenoxicam
3. Xefocam
4. Xefo
5. Lorcam
6. Lornoxicamum
7. Ro 13-9297
8. Lornoxicamum [inn-latin]
9. Taigalor
10. Safem
11. Telos
12. Lorcam (tn)
13. Cltx
14. Lornoxicam (xefo)
15. Ccris 8589
16. Nsc-759620
17. Ro-13-9297
18. 6-chloro-4-hydroxy-2-methyl-1,1-dioxo-n-pyridin-2-ylthieno[2,3-e]thiazine-3-carboxamide
19. 6-chloro-4-hydroxy-2-methyl-n-(pyridin-2-yl)-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide
20. Chebi:31783
21. Er09126g7a
22. Chlortenoxicam;ro 13-9297;ts110
23. Ro-139297
24. 2h-thieno(2,3-e)-1,2-thiazine-3-carboxamide, 6-chloro-4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
25. 6-chloro-4-hydroxy-2-methyl-n-pyridin-2-yl-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide
26. 70374-27-5
27. 6-chloro-4-hydroxy-2-methyl-1,1-dioxo-n-(pyridin-2-yl)-2h-1$l^{6},5,2-thieno[2,3-e][1$l^{6},2]thiazine-3-carboxamide
28. Smr000550483
29. Lornoxicam [usan:inn:ban]
30. Brn 1039965
31. Unii-er09126g7a
32. 2h-thieno[2,3-e]-1,2-thiazine-3-carboxamide, 6-chloro-4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
33. Mfcd00866163
34. Lornoxicam [mi]
35. Lornoxicam [inn]
36. Lornoxicam [jan]
37. Lornoxicam [usan]
38. Lornoxicam [mart.]
39. Lornoxicam [who-dd]
40. Schembl35101
41. Schembl35102
42. 6-chloro-4-hydroxy-2-methyl-n-2-pyridinyl-2h-thieno[2,3-e]-1,2-thiazine-3-carboxamide 1,1-dioxide
43. 6-chloro-4-hydroxy-2-methyl-n-2-pyridyl-2h-thieno(2,3-e)-1,2-thiazine-3-carboxamide 1,1-dioxide
44. Mls001165721
45. Mls001304721
46. Mls006010084
47. Lornoxicam (jan/usan/inn)
48. Schembl1650239
49. Chembl1569487
50. Chembl3188235
51. Lornoxicam, >=98% (hplc)
52. Bdbm92331
53. Hms2231b07
54. Hms3264i20
55. Hms3655n13
56. Hms3887c17
57. Pharmakon1600-01502302
58. Ts110
59. Albb-014101
60. Bcp04666
61. Hy-b0367
62. Zinc5318420
63. Nsc759620
64. S2047
65. Stl504715
66. Ts-110
67. Zinc49089868
68. Akos000281810
69. Akos026750113
70. Zinc100015491
71. Ab07518
72. Ac-4675
73. Bcp9000860
74. Ccg-213023
75. Db06725
76. Ks-1080
77. Nsc 759620
78. Ncgc00246566-01
79. Ncgc00346667-01
80. Ncgc00346667-02
81. 6-chloro-4-hydroxy-2-methyl-3-(2-pyridylcarbamoyl)-2h-thieno[2,3-e]-1,2-thiazine-1,1-dioxide
82. Hn-10000
83. Bcp0726000036
84. Ft-0630797
85. Ft-0670854
86. L0239
87. Sw220125-1
88. C76243
89. D01866
90. Ab00876293_04
91. Ab00876293_06
92. 374l399
93. A836864
94. Sr-01000799148
95. Q-101309
96. Q2734874
97. Sr-01000799148-3
98. (3e)-6-chloro-3-[hydroxy(pyridin-2-ylamino)methylene]- 2-methyl-2,3-dihydro-4h-thieno[2,3-e][1,2] Thiazin- 4 - One 1,1-dioxide
99. (3e)-6-chloro-3-[hydroxyl(pyridin-2-ylamino)methylene]-2-methyl-2,3-dihydro-4h-thieno[2,3-e][1,2]thiazin-4-one 1,1-dioxide
100. (3z)-6-chloranyl-2-methyl-1,1-bis(oxidanylidene)-3-[oxidanyl-(pyridin-2-ylamino)methylidene]thieno[2,3-e][1,2]thiazin-4-one
101. (3z)-6-chloro-3-[hydroxy-(2-pyridinylamino)methylidene]-2-methyl-1,1-dioxo-4-thieno[2,3-e]thiazinone
102. 2h-thieno(3,2-e)-1,2-thiazine-3-carboxamide, 6-chloro-4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
103. 6-chloro-4-hydroxy-2-methyl-1,1-dioxo-n-(pyridin-2-yl)-2h-1
104. E?-thieno[2,3-e][1,2]thiazine-3-carboxamide
105. 6-chloro-4-hydroxy-2-methyl-n-(2-pyridyl)-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide
106. 6-chloro-4-hydroxy-2-methyl-n-(pyridin-2-yl)-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide1,1-dioxide
107. 6-chloro-4-hydroxy-2-methyl-n-pyridin-2-yl-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide (lornoxicam)
108. 6-chloro-4-hydroxy-2-methyl-n-pyridin-2-yl-2h-thieno[2,3-e][1,2]thiazine-3-carboxamide-1,1-dioxide
| Molecular Weight | 371.8 g/mol |
|---|---|
| Molecular Formula | C13H10ClN3O4S2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 370.9801258 g/mol |
| Monoisotopic Mass | 370.9801258 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 634 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of acute mild to moderate pain, as well as pain and inflammation of the joints caused by certain types of rheumatic diseases.
Lornoxicam is a non-steroidal anti-inflammatory drug (NSAID) that belongs to the oxicam class. As with other NSAIDS, lornoxicam is a potent inhibitor of the cyclooxgenase enzymes, which are responsible for catalyzing the formation of prostaglandins (act as messenger molecules in the process of inflammation) and thromboxane from arachidonic acid. Unlike some NSAIDS, lornoxicam's inhibition of cyclooxygenase does not lead to an increase in leukotriene formation, meaning that arachidonic acid is not moved to the 5-lipoxygenase cascade, resulting in the minimization of the risk of adverse events.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AC - Oxicams
M01AC05 - Lornoxicam
Absorption
Lornoxicam is absorbed rapidly and almost completely from the GI tract (90-100%).
Lornoxicam is metabolized completely by cyp 2C9 with the principal metabolite being 5'-hydroxy-lornoxicam and only negligible amounts of intact lornoxicam are excreted unchanged in the urine. Approximately 2/3 of the drug is eliminated via the liver and 1/3 via the kidneys in the active form.
Lornoxicam has known human metabolites that include 5'-Hydroxylornoxicam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
3-5 hours
Like other NSAIDS, lornoxicam's anti-inflammatory and analgesic activity is related to its inhibitory action on prostaglandin and thromboxane synthesis through the inhibition of both COX-1 and COX-2. This leads to the reduction of inflammation, pain, fever, and swelling, which are mediated by prostaglandins. However, the exact mechanism of lornoxicam, like that of the other NSAIDs, has not been fully determined.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

ABOUT THIS PAGE
100
PharmaCompass offers a list of Lornoxicam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lornoxicam manufacturer or Lornoxicam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lornoxicam manufacturer or Lornoxicam supplier.
PharmaCompass also assists you with knowing the Lornoxicam API Price utilized in the formulation of products. Lornoxicam API Price is not always fixed or binding as the Lornoxicam Price is obtained through a variety of data sources. The Lornoxicam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A xefocam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of xefocam, including repackagers and relabelers. The FDA regulates xefocam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. xefocam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of xefocam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A xefocam supplier is an individual or a company that provides xefocam active pharmaceutical ingredient (API) or xefocam finished formulations upon request. The xefocam suppliers may include xefocam API manufacturers, exporters, distributors and traders.
click here to find a list of xefocam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A xefocam DMF (Drug Master File) is a document detailing the whole manufacturing process of xefocam active pharmaceutical ingredient (API) in detail. Different forms of xefocam DMFs exist exist since differing nations have different regulations, such as xefocam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A xefocam DMF submitted to regulatory agencies in the US is known as a USDMF. xefocam USDMF includes data on xefocam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The xefocam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of xefocam suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The xefocam Drug Master File in Japan (xefocam JDMF) empowers xefocam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the xefocam JDMF during the approval evaluation for pharmaceutical products. At the time of xefocam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of xefocam suppliers with JDMF on PharmaCompass.
A xefocam written confirmation (xefocam WC) is an official document issued by a regulatory agency to a xefocam manufacturer, verifying that the manufacturing facility of a xefocam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting xefocam APIs or xefocam finished pharmaceutical products to another nation, regulatory agencies frequently require a xefocam WC (written confirmation) as part of the regulatory process.
click here to find a list of xefocam suppliers with Written Confirmation (WC) on PharmaCompass.
xefocam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of xefocam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right xefocam GMP manufacturer or xefocam GMP API supplier for your needs.
A xefocam CoA (Certificate of Analysis) is a formal document that attests to xefocam's compliance with xefocam specifications and serves as a tool for batch-level quality control.
xefocam CoA mostly includes findings from lab analyses of a specific batch. For each xefocam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
xefocam may be tested according to a variety of international standards, such as European Pharmacopoeia (xefocam EP), xefocam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (xefocam USP).
