
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
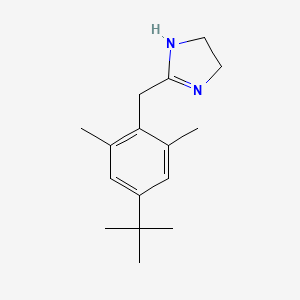

1. 2-(4'-tert-butyl-2',6'-dimethylphenylmethyl)imidazoline
2. Amidrin
3. Balkis
4. Chlorohist-la
5. Decongest
6. Espa-rhin
7. Gelonasal
8. Idasal
9. Idril N
10. Imidin
11. Nasan
12. Nasengel Al
13. Nasengel Ratiopharm
14. Nasenspray Al
15. Nasenspray Ratiopharm
16. Nasentropfen Al
17. Nasentropfen Ratiopharm
18. Novorin
19. Otradrops
20. Otraspray
21. Otriven
22. Otrivin
23. Otrivin Mentol
24. Rapako
25. Schnupfen Endrine
26. Snup
27. Stas
28. Xylometazoline Hydrochloride
29. Xylometazoline Monohydrochloride
1. 526-36-3
2. Xylomethazoline
3. Otrivine
4. Otrivin
5. Otriven
6. Otrix
7. 2-[(4-tert-butyl-2,6-dimethylphenyl)methyl]-4,5-dihydro-1h-imidazole
8. Balminil
9. 2-(4-(tert-butyl)-2,6-dimethylbenzyl)-4,5-dihydro-1h-imidazole
10. 2-(4-tert-butyl-2,6-dimethyl-benzyl)-4,5-dihydro-1h-imidazole
11. Ba-11391
12. 2-(4-tert-butyl-2,6-dimethylbenzyl)-2-imidazoline
13. Wpy40fth8k
14. Chembl312448
15. 2-imidazoline, 2-(4-tert-butyl-2,6-dimethylbenzyl)-
16. Ba 11391; Otriven; Otrivin
17. Chebi:10082
18. 1h-imidazole, 2-((4-(1,1-dimethylethyl)-2,6-dimethylphenyl)methyl)-4,5-dihydro-
19. Xylometazoline (inn)
20. Xilometazolina
21. 2-(4-tert-butyl-2,6-dimethylbenzyl)-4,5-dihydro-1h-imidazole
22. 1h-imidazole, 2-[[4-(1,1-dimethylethyl)-2,6-dimethylphenyl]methyl]-4,5-dihydro-
23. Xylometazoline [inn]
24. Xylometazolinum
25. Xylometazoline [inn:ban]
26. Xylometazolinum [inn-latin]
27. Xilometazolina [inn-spanish]
28. Ncgc00016101-02
29. Einecs 208-390-6
30. Unii-wpy40fth8k
31. Ba 11391
32. Cas-1218-35-5
33. Brn 0180524
34. Balminil (tn)
35. Spectrum_000382
36. 2-[(4-tert-butyl-2,6-dimethylphenyl)methyl]-4,5-dihydro-1h-imidazole;hydrochloride
37. Prestwick0_000223
38. Prestwick1_000223
39. Prestwick2_000223
40. Prestwick3_000223
41. Spectrum2_000945
42. Spectrum3_000586
43. Spectrum4_000382
44. Spectrum5_001469
45. Lopac-x-6000
46. Xylometazoline [mi]
47. Lopac0_001269
48. Schembl34087
49. Bspbio_000265
50. Bspbio_002032
51. Gtpl517
52. Kbiogr_000903
53. Kbioss_000862
54. 5-23-07-00109 (beilstein Handbook Reference)
55. Divk1c_000158
56. Xylometazoline [vandf]
57. Spbio_000910
58. Spbio_002186
59. Bpbio1_000293
60. Xylometazoline [who-dd]
61. Dtxsid8046957
62. Bdbm30703
63. Cid_5282386
64. Kbio1_000158
65. Kbio2_000862
66. Kbio2_003430
67. Kbio2_005998
68. Kbio3_001532
69. Zinc57534
70. Ninds_000158
71. 2-{[4-(tert-butyl)-2,6-dimethylphenyl]methyl}-2-imidazoline
72. Hms3604m22
73. S5854
74. Stk558658
75. Akos000115430
76. Ccg-205342
77. Db06694
78. Ks-5071
79. Sdccgsbi-0051235.p004
80. 2-[[4-(1,1-dimethylethyl)-2,6-dimethylphenyl]methyl]-4,5-dihydro-1h-imidazole
81. Idi1_000158
82. Ncgc00016101-01
83. Ncgc00016101-03
84. Ncgc00016101-04
85. Ncgc00016101-05
86. Ncgc00016101-06
87. Ncgc00016101-07
88. Ncgc00016101-09
89. Ncgc00016101-18
90. Ncgc00024281-03
91. Sbi-0051235.p003
92. Db-052151
93. Ft-0603452
94. En300-02385
95. Vu0239751-6
96. C07913
97. D08684
98. Q31030
99. Ab00053567_11
100. Ab00053567_12
101. 526b363
102. L001171
103. Brd-k08356259-003-05-6
104. Brd-k08356259-003-15-5
105. F1371-0282
106. 2-(4-tert-butyl-2,6-dimethyl-benzyl)-2-imidazoline;hydrochloride
107. 2-(4-tert-butyl-2,6-dimethylbenzyl)-4,5-dihydro-1h-imidazole #
108. 2-[(2,6-dimethyl-4-tert-butyl-phenyl)methyl]-4,5-dihydroimidazole
109. 2-[(4-t-butyl-2,6-dimethylphenyl)methyl]-4,5-dihydro-1h-imidazole
110. 2-[(4-t-butyl-2,6-dimethylphenyl)methyl]4,5dihydro-1h-imidazole
111. 2-[(4-tert-butyl-2,6-dimethyl-phenyl)methyl]-4,5-dihydro-1h-imidazole;hydrochloride
| Molecular Weight | 244.37 g/mol |
|---|---|
| Molecular Formula | C16H24N2 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| Exact Mass | 244.193948774 g/mol |
| Monoisotopic Mass | 244.193948774 g/mol |
| Topological Polar Surface Area | 24.4 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 302 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Xylometazoline is indicated for the temporary relief of nasal congestion due to cold, hay fever or other respiratory allergies.
Xylometazoline is a sympathomimetic agent that causes vasoconstriction of the nasal mucosa. In one study comprising subjects with nasal congestion associated with the common cold, the median time of onset of subjective relief of nasal congestion was about 1.7 minutes and the time of subjective peak relief of nasal congestion was 30 minutes. Previous studies reported rebound swelling, rebound nasal congestion, rhinitis medicamentosa, and shorter duration of decongestant effect from the long-term use of xylometazoline in healthy volunteers, suggesting that the drug is most effective if used temporarily. An early _in vitro_ study demonstrated xylometazoline to exert anti-oxidant actions, where it inhibited microsomal lipid peroxidation and mediated hydroxyl radical scavenging activity. This suggests that xylometazoline has a beneficial effect against oxidants, which play a role in tissue damage in inflammation.
Nasal Decongestants
Drugs designed to treat inflammation of the nasal passages, generally the result of an infection (more often than not the common cold) or an allergy related condition, e.g., hay fever. The inflammation involves swelling of the mucous membrane that lines the nasal passages and results in inordinate mucus production. The primary class of nasal decongestants are vasoconstrictor agents. (From PharmAssist, The Family Guide to Health and Medicine, 1993) (See all compounds classified as Nasal Decongestants.)
R01AA07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AA - Sympathomimetics, plain
R01AA07 - Xylometazoline
R - Respiratory system
R01 - Nasal preparations
R01A - Decongestants and other nasal preparations for topical use
R01AB - Sympathomimetics, combinations excl. corticosteroids
R01AB06 - Xylometazoline
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GA - Sympathomimetics used as decongestants
S01GA03 - Xylometazoline
Absorption
No information is available on xylometazoline pharmacokinetics.
Route of Elimination
No information is available on xylometazoline pharmacokinetics.
Volume of Distribution
No information is available on xylometazoline pharmacokinetics.
Clearance
No information is available on xylometazoline pharmacokinetics.
No information is available on xylometazoline pharmacokinetics.
No information is available on xylometazoline pharmacokinetics.
Nasal congestion is caused by various etiologies, such as rhinosinusitis and allergic or non-allergic rhinitis, leading to congestion of the venous sinusoids lining the nasal mucosa. Activation of -adrenergic receptors leads to vasoconstriction of the blood vessels of the nasal mucosa and resumption of nasal airflow. As the most abundantly expressed in the human nasal mucosa, 1A- and 2B-adrenoceptors may play the most important role in vasoconstriction of the human nasal mucosa. Xylometazoline is a more selective agonist at 2B-adrenoceptors, with affinity at 1A-, 2A-, 2C-, 1B-, and 1D-adrenoceptors. Xylometazoline decreases nasal resistance during inspiration and expiration and increases the volume of nasal airflow. Compared to [oxymetazoline], another imidazoline nasal decongestant, xylometazoline had a slightly faster onset of action although they had a similar duration of action. In one study, subjects with nasal congestion reported relief of earache and sore throat in addition to nasal decongestion: it is speculated that oxymetazoline mediates this effect by causing vasoconstriction of the nasal mucosa that contains the venous sinuses and nasal decongestion allows breathing through the nose, providing relief from sore throat caused by mouth breathing that dries and irritates the throat.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
42
PharmaCompass offers a list of Xylometazoline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Xylometazoline manufacturer or Xylometazoline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Xylometazoline manufacturer or Xylometazoline supplier.
PharmaCompass also assists you with knowing the Xylometazoline API Price utilized in the formulation of products. Xylometazoline API Price is not always fixed or binding as the Xylometazoline Price is obtained through a variety of data sources. The Xylometazoline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Xylometazoline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Xylometazoline, including repackagers and relabelers. The FDA regulates Xylometazoline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Xylometazoline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Xylometazoline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Xylometazoline supplier is an individual or a company that provides Xylometazoline active pharmaceutical ingredient (API) or Xylometazoline finished formulations upon request. The Xylometazoline suppliers may include Xylometazoline API manufacturers, exporters, distributors and traders.
click here to find a list of Xylometazoline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Xylometazoline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Xylometazoline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Xylometazoline GMP manufacturer or Xylometazoline GMP API supplier for your needs.
A Xylometazoline CoA (Certificate of Analysis) is a formal document that attests to Xylometazoline's compliance with Xylometazoline specifications and serves as a tool for batch-level quality control.
Xylometazoline CoA mostly includes findings from lab analyses of a specific batch. For each Xylometazoline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Xylometazoline may be tested according to a variety of international standards, such as European Pharmacopoeia (Xylometazoline EP), Xylometazoline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Xylometazoline USP).
