
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
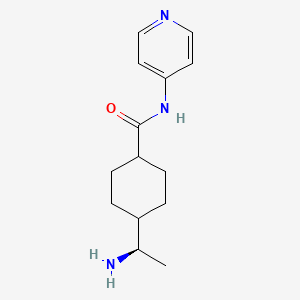

1. N-(4-pyridyl)-4-(1-aminoethyl)cyclohexanecarboxamide
2. Y 27632
3. Y 27632, (4(r)-trans)-isomer
4. Y 27632, (4(s)-trans)-isomer
5. Y 27632, (trans)-isomer
6. Y 27632, Dihydrochloride, (4(r)-trans)-isomer
7. Y-27632
8. Y27632
1. 146986-50-7
2. Y-27632
3. Y27632
4. Y 27632
5. Cyclohexanecarboxamide, 4-[(1r)-1-aminoethyl]-n-4-pyridinyl-, Trans-
6. 4-[(1r)-1-aminoethyl]-n-pyridin-4-ylcyclohexane-1-carboxamide
7. Y-27632, Free Base
8. 4-[(1r)-1-aminoethyl]-n-(pyridin-4-yl)cyclohexane-1-carboxamide
9. 0x370rop6h
10. Chebi:75393
11. (r)-trans-4-(1-aminoethyl)-n-(4-pyridyl) Cyclohexanecarboxamide
12. 521059-79-0
13. (1r,4r)-4-((r)-1-aminoethyl)-n-(pyridin-4-yl)cyclohexanecarboxamide
14. (r)-(+)-trans-n-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide
15. Ximelegatran
16. (r)-4-(1-aminoethyl)-n-(pyridin-4-yl)cyclohexanecarboxamide
17. Trans-4-((r)-1-aminoethyl)-n-(pyridin-4-yl)cyclohexanecarboxamide
18. Y27
19. Unii-0x370rop6h
20. (+)-(r)-trans-4-(1-aminoethyl)-n-(4-pyridyl)cyclohexanecarboxamide
21. [+]-[r]-trans-4-[1-aminoethyl]-n-[4-pyridyl]cyclohexanecarboxamide
22. Cyclohexanecarboxamide, 4-(1-aminoethyl)-n-4-pyridinyl-, [4(r)-trans]-
23. 2etr
24. 2gnf
25. 2gnj
26. Cyclohexanecarboxamide, 4-((1r)-1-aminoethyl)-n-4-pyridinyl-, Trans-
27. Cyclohexanecarboxamide, 4-(1-aminoethyl)-n-4-pyridinyl-, (4(r)-trans)-
28. Iyoztvgmewjpkr-vomcllrmsa-n
29. 1q8t
30. Biomolki_000071
31. Y27632 Dihydrochloride
32. Biomolki2_000075
33. Cbiol_001962
34. Bspbio_001234
35. Kbiogr_000574
36. Kbioss_000574
37. Schembl598993
38. Chembl559147
39. Gtpl5290
40. Rock Inhibitor, Y-27632
41. Schembl1980406
42. Schembl7804373
43. Chembl1083134
44. Chembl1188380
45. Dtxsid7043740
46. Schembl13970844
47. Schembl18935698
48. Y-27632dihydrochloride
49. Bdbm14029
50. Bdbm86729
51. Chebi:92773
52. Kbio2_000574
53. Kbio2_003142
54. Kbio2_005710
55. Kbio3_001027
56. Kbio3_001028
57. 4-[(1r)-1-aminoethyl]-n-(4-pyridyl)cyclohexanecarboxamide
58. Bcpp000008
59. Bio1_000248
60. Bio1_000737
61. Bio1_001226
62. Bio2_000457
63. Bio2_000937
64. Hms1362n15
65. Hms1792n15
66. Hms1990n15
67. Hms3403n15
68. Y 27632 [who-dd]
69. Bdbm50319631
70. Nsc751297
71. S6390
72. (1r,4r)-4-[(1r)-1-aminoethyl]-n-(pyridin-4-yl)cyclohexane-1-carboxamide
73. Akos026750241
74. Akos028109253
75. Am81233
76. Ccg-204428
77. Cs-0131
78. Db08756
79. Nsc-751297
80. Idi1_002212
81. Smp2_000199
82. Ncgc00092276-02
83. Ncgc00092276-03
84. Ncgc00092276-04
85. Ncgc00092276-05
86. Ncgc00092276-06
87. Ncgc00092276-07
88. Ncgc00092276-08
89. Ncgc00092276-09
90. Ncgc00092276-10
91. Ncgc00387412-04
92. As-77772
93. Hy-10071
94. J888.082d
95. Cas_146986-50-7
96. Ns00073644
97. A25646
98. J-008296
99. J-525164
100. Q6584634
101. Brd-k44084986-001-03-9
102. Brd-k44084986-300-02-7
103. (r)-trans-4-(1-aminoethyl)-n-(4-pyridyl)cyclohexanecarboxamide
104. Trans-4-[(1r)-1-aminoethyl]-n-(pyridin-4-yl)cyclohexanecarboxamide
105. Trans-4-[(1r)-1-aminoethyl]-n-pyridin-4-ylcyclohexanecarboxamide
106. (1r,4r)-4-((r)-1-aminoethyl)-n-(pyridin-4-yl)cyclohexane-1-carboxamide
107. (r)-(+)-trans-4-(1-aminoethyl)-n-(4-pyridyl)cyclohexanecarboxamide
108. 4.beta.-((1r)-1-aminoethyl)-n-(4-pyridinyl)cyclohexane-1.alpha.-carboxamide
109. Cyclohexanecarboxamide, 4-[(1r)-1-aminoethyl]-n-4-pyridinyl-, Trans- (9ci)
110. Trans-4-[(1r)-1-aminoethyl]-n-4-pyridinylcyclohexanecar Boxamide Dihydrochloride
| Molecular Weight | 247.34 g/mol |
|---|---|
| Molecular Formula | C14H21N3O |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 68 |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 268 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?