
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
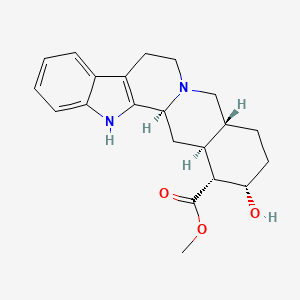

1. Aphrodine Hydrochloride
2. Aphrodyne
3. Corynanthine
4. Corynanthine Tartrate
5. Hydrochloride, Aphrodine
6. Hydrochloride, Yohimbine
7. Pluriviron
8. Rauhimbine
9. Rauwolscine
10. Tartrate, Corynanthine
11. Yocon
12. Yohimbin Spiegel
13. Yohimbine Houd
14. Yohimbine Hydrochloride
15. Yohimex
1. Yohimbin
2. 146-48-5
3. Quebrachin
4. Quebrachine
5. Corynine
6. Aphrodine
7. Yohimbic Acid Methyl Ester
8. Aphrosol
9. (+)-yohimbine
10. Johimbin
11. Actibine
12. Yohimbinum
13. 17-hydroxyyohimban-16-carboxylic Acid Methyl Ester
14. Yohimban-16-carboxylic Acid, 17-hydroxy-, Methyl Ester, (16alpha,17alpha)-
15. Chembl15245
16. 2y49vwd90q
17. 17alpha-hydroxyyohimban-16alpha-carboxylic Acid Methyl Ester
18. Chebi:10093
19. (16alpha,17alpha)-17-hydroxyyohimban-16-carboxylic Acid Methyl Ester
20. Methyl 17alpha-hydroxyyohimban-16alpha-carboxylate
21. Dsstox_cid_20130
22. Dsstox_rid_79446
23. Dsstox_gsid_40130
24. Methyl (1s,15r,18s,19r,20s)-18-hydroxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate
25. Methyl (1s,15r,18s,19r,20s)-18-hydroxy-3,13-diazapentacyclo[11.8.0.0^{2,10}.0^{4,9}.0^{15,20}]henicosa-2(10),4,6,8-tetraene-19-carboxylate
26. Yohimbine Chloride
27. Chembl537669
28. Yohimbine (dcf)
29. Cas-146-48-5
30. Amberlite Cg 400
31. Amberlite Cg-400
32. Methyl Hydroxy[?]carboxylate
33. Einecs 205-672-0
34. Brn 0097276
35. Trans-quinolizidine Yohimbine
36. Unii-2y49vwd90q
37. Yohimbe Bark
38. Yohimbe Hydrochloride
39. Ccris 9415
40. Sr-01000075297
41. Benz[g]indolo[2,3-a]quinolizine, Yohimban-16-carboxylic Acid Deriv.
42. Yohimbehydrochloride
43. Yohimbe Bark Extract
44. 37247-87-3
45. Nsc19509
46. Yohimbine [mi]
47. Methyl (2s,13bs,14as,1r,4ar)-2-hydroxy-1,2,3,4,5,8,14,13b,14a,4a-decahydrobenz O[1,2-g]indolo[2,3-a]quinolizinecarboxylate
48. Prestwick0_000584
49. Prestwick1_000584
50. Prestwick2_000584
51. Prestwick3_000584
52. Yohimban-16-alpha-carboxylic Acid, 17-alpha-hydroxy-, Methyl Ester
53. Yohimbe Extract Yohimbine
54. Yohimbine [vandf]
55. Yohimbinum [hpus]
56. Cid_6169
57. Cid_8969
58. Yohimbine [who-dd]
59. Lopac0_001210
60. Schembl33954
61. Bspbio_000428
62. Bspbio_001236
63. Gtpl102
64. Kbiogr_000576
65. Kbioss_000576
66. 4-25-00-01237 (beilstein Handbook Reference)
67. Mls000728591
68. Spbio_002647
69. Bpbio1_000472
70. Yohimbol-16alpha-carboxylic Acid, Methyl Ester (6ci)
71. Dtxsid9040130
72. Bcbcmap01_000032
73. Kbio2_000576
74. Kbio2_003144
75. Kbio2_005712
76. Kbio3_001031
77. Kbio3_001032
78. Bio1_000455
79. Bio1_000944
80. Bio1_001433
81. Bio2_000458
82. Bio2_000938
83. Hms1362n17
84. Hms1792n17
85. Hms1990n17
86. Hms2089g19
87. Hms2234c18
88. Zinc3860825
89. Tox21_110019
90. Bdbm50013515
91. Bdbm50203564
92. Mfcd00005093
93. Akos015902024
94. Tox21_110019_1
95. Yohimban-16-.alpha.-carboxylic Acid, 17-.alpha.-hydroxy-, Methyl Ester
96. Yohimban-16alpha-carboxylic Acid, 17alpha-hydroxy-, Methyl Ester (8ci)
97. Ccg-205284
98. Cs-5173
99. Db01392
100. Gs-5751
101. Sdccgsbi-0051177.p002
102. Idi1_002213
103. Mrf-0000020
104. Smp1_000320
105. Ncgc00013260-01
106. Ncgc00025018-05
107. Ncgc00025018-06
108. Ncgc00025018-07
109. Ncgc00025018-10
110. Ncgc00025018-11
111. Ncgc00025018-17
112. Hy-12715
113. Smr000470778
114. 17a-hydroxy-16a-methoxycarbonyl-yohimbane
115. 65y190
116. C09256
117. D08685
118. H10057
119. Q412226
120. Sr-01000075297-5
121. Brd-k35586044-001-02-6
122. Brd-k35586044-003-03-0
123. Brd-k35586044-003-11-3
124. 17.alpha.-hydroxy-20-.alpha.-yohimban-16-.beta.-carboxylic Acid, Methyl Ester
125. Yohimban-16-carboxylic Acid, 17-hydroxy-, Methyl Ester, (16alpha,17alpha)- (9ci)
126. (1r,2s,4ar,13bs,14as)-2-hydroxy-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydro-indolo[2'',3'':3,4]pyrido[1,2-b]isoquinoline-1-carboxylic Acid Methyl Ester Hydrochloride
127. 103834-06-6
| Molecular Weight | 354.4 g/mol |
|---|---|
| Molecular Formula | C21H26N2O3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 354.19434270 g/mol |
| Monoisotopic Mass | 354.19434270 g/mol |
| Topological Polar Surface Area | 65.6 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 555 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated as a sympatholytic and mydriatic. Impotence has been successfully treated with yohimbine in male patients with vascular or diabetic origins and psychogenic origins.
Yohimbine is an indolalkylamine alkaloid with chemical similarity to reserpine. Yohimbine blocks presynaptic alpha-2 adrenergic receptors. Its action on peripheral blood vessels resembles that of reserpine, though it is weaker and of short duration. Yohimbine's peripheral autonomic nervous system effect is to increase parasympathetic (cholinergic) and decrease sympathetic (adrenergic) activity. It is to be noted that in male sexual performance, erection is linked to cholinergic activity and to alpha-2 adrenergic blockade which may theoretically result in increased penile inflow, decreased penile outflow or both. Yohimbine exerts a stimulating action on the mood and may increase anxiety. Such actions have not been adequately studied or related to dosage although they appear to require high doses of the drug. Yohimbine has a mild anti-diuretic action, probably via stimulation of hypothalmic center and release of posterior pituitary hormone. Reportedly Yohimbine exerts no significant influence on cardiac stimulation and other effects mediated by (beta)-adrenergic receptors. Its effect on blood pressure, if any, would be to lower it; however, no adequate studies are at hand to quantitate this effect in terms of Yohimbine dosage.
Adrenergic alpha-2 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC ALPHA-2 RECEPTORS. (See all compounds classified as Adrenergic alpha-2 Receptor Antagonists.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
Mydriatics
Agents that dilate the pupil. They may be either sympathomimetics or parasympatholytics. (See all compounds classified as Mydriatics.)
G04BE04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BE - Drugs used in erectile dysfunction
G04BE04 - Yohimbine
Absorption
Rapidly absorbed following oral administration. Bioavailability is highly variable, ranging from 7 to 87% (mean 33%).
Yohimbine appears to undergo extensive metabolism in an organ of high flow such as the liver or kidney, however, the precise metabolic fate of yohimbine has not been fully determined.
Elimination half-life is approximately 36 minutes.
Yohimbine is a pre-synaptic alpha 2-adrenergic blocking agent. The exact mechanism for its use in impotence has not been fully elucidated. However, yohimbine may exert its beneficial effect on erectile ability through blockade of central alpha 2-adrenergic receptors producing an increase in sympathetic drive secondary to an increase in norepinephrine release and in firing rate of cells in the brain noradrenergic nuclei. Yohimbine-mediated norepinephrine release at the level of the corporeal tissues may also be involved. In addition, beneficial effects may involve other neurotransmitters such as dopamine and serotonin and cholinergic receptors.
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
42
PharmaCompass offers a list of Yohimbine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Yohimbine manufacturer or Yohimbine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Yohimbine manufacturer or Yohimbine supplier.
PharmaCompass also assists you with knowing the Yohimbine API Price utilized in the formulation of products. Yohimbine API Price is not always fixed or binding as the Yohimbine Price is obtained through a variety of data sources. The Yohimbine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Yohimbine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Yohimbine, including repackagers and relabelers. The FDA regulates Yohimbine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Yohimbine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Yohimbine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Yohimbine supplier is an individual or a company that provides Yohimbine active pharmaceutical ingredient (API) or Yohimbine finished formulations upon request. The Yohimbine suppliers may include Yohimbine API manufacturers, exporters, distributors and traders.
click here to find a list of Yohimbine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Yohimbine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Yohimbine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Yohimbine GMP manufacturer or Yohimbine GMP API supplier for your needs.
A Yohimbine CoA (Certificate of Analysis) is a formal document that attests to Yohimbine's compliance with Yohimbine specifications and serves as a tool for batch-level quality control.
Yohimbine CoA mostly includes findings from lab analyses of a specific batch. For each Yohimbine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Yohimbine may be tested according to a variety of international standards, such as European Pharmacopoeia (Yohimbine EP), Yohimbine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Yohimbine USP).
