
Synopsis
Synopsis
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
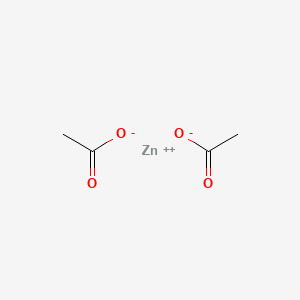

1. Anhydrous Zinc Acetate
2. Galzin
3. Zinc Acetate Anhydrous
4. Zinc Acetate Dihydrate
5. Zinc Acetate, Anhydrous
1. 557-34-6
2. Zinc Diacetate
3. Zinc(ii) Acetate
4. Dicarbomethoxyzinc
5. Acetic Acid, Zinc Salt
6. Zinc Acetate Anhydrous
7. Acetic Acid, Zinc(ii) Salt
8. Zinc Di(acetate)
9. Zn(oac)2
10. Zinc Acetate Basic
11. H2zey72pme
12. Chebi:62984
13. Acetic Acid, Zinc Salt (2:1)
14. Mfcd00012454
15. Nsc-75801
16. 82279-57-0
17. Zinc Acetate, Anhydrous
18. Siltex Cl 4
19. Ccris 3471
20. Hsdb 1043
21. Einecs 209-170-2
22. Unii-h2zey72pme
23. Nsc 75801
24. Zinc;diacetate
25. Ai3-04465
26. Zinc (ii) Acetate
27. Zincum Aceticum
28. Anhydrous Zinc Acetate
29. Zinc Acetate Carrageenan
30. Zn(ii)ac2
31. Za/cg
32. Schembl51
33. Zinc Acetate [mi]
34. Zinc Acetate 35% 40m
35. (ch3co2)2zn
36. Dsstox_cid_18770
37. Dsstox_rid_79392
38. Zinc Acetate [inci]
39. Dsstox_gsid_38770
40. Zinc Acetate [who-dd]
41. Zincum Aceticum [hpus]
42. Chembl1200928
43. Dtxsid8038770
44. Zinc Acetate, Trace Metals Grade
45. Tox21_302016
46. Zinc Acetate Anhydrous [hsdb]
47. Akos015837576
48. Nanodtpa Component Zinc Acetate
49. Anhydrous Zinc Acetate [mart.]
50. Db14487
51. Ncgc00255475-01
52. Cas-557-34-6
53. E650
54. Sy010404
55. Cs-0013863
56. Ft-0689089
57. Z0044
58. E70002
59. Nano-dtpa Capsule Component Zinc Acetate
60. Nanodtpa Zn-dtpa Component Zinc Acetate
61. A918239
62. Q204639
| Molecular Weight | 183.5 g/mol |
|---|---|
| Molecular Formula | C4H6O4Zn |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 181.955750 g/mol |
| Monoisotopic Mass | 181.955750 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Galzin |
| PubMed Health | Zinc Acetate (By mouth) |
| Drug Classes | Zinc Supplement |
| Drug Label | Zinc acetate as the dihydrate is a salt of zinc used to inhibit the absorption of copper in patients with Wilson's disease. Its structural formula is:C4H6O4Zn2H2OM.W. 219.51.Zinc acetate occurs as white crystals or granules, freely sol... |
| Active Ingredient | Zinc acetate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 25mg zinc; eq 50mg zinc |
| Market Status | Prescription |
| Company | Teva |
| 2 of 2 | |
|---|---|
| Drug Name | Galzin |
| PubMed Health | Zinc Acetate (By mouth) |
| Drug Classes | Zinc Supplement |
| Drug Label | Zinc acetate as the dihydrate is a salt of zinc used to inhibit the absorption of copper in patients with Wilson's disease. Its structural formula is:C4H6O4Zn2H2OM.W. 219.51.Zinc acetate occurs as white crystals or granules, freely sol... |
| Active Ingredient | Zinc acetate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 25mg zinc; eq 50mg zinc |
| Market Status | Prescription |
| Company | Teva |
/Zinc acetate USP is used as/ an astringent in low concentrations and an irritant at high concentrations. It also has mild antibacterial actions similar to those of zinc sulfate. When applied to cuts, it exerts styptic action. After oral ingestion ... .
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 718
THERAP CAT: Styptic, astringent
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1810
THERAP CAT (VET): Antiseptic, astringent, protective (topical)
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1810
Orally administered zinc inhibits copper absorption through the intestine. Oral zinc /acetate/ therapy /for Wilson's disease/ is probably a safe and effective maintenance treatment for patients who were initially treated with chelating agents, and seems to be an appropriate first-line therapy for presymptomatic and pregnant patients. Combination drug therapy of a chelating agent and zinc has no advantages when compared with zinc only, during maintenance therapy.
Chang, L.W. (ed.). Toxicology of Metals. Boca Raton, FL: Lewis Publishers, 1996, p. 377
For more Therapeutic Uses (Complete) data for ZINC ACETATE (9 total), please visit the HSDB record page.
Zinc does appear in breast milk and zinc-induced copper deficiency in the nursing baby may occur. Therefore, it is recommended that women on zinc therapy not nurse their babies.
US Natl Inst Health; DailyMed. Current Medication Information for Galzin - Zinc Acetate (April 2006). Available from, as of August 26, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1081
Transient elevations in serum amylase, lipase, and alkaline phosphatase have been observed in patients with Wilson's disease receiving zinc acetate therapy (25 or 50 mg elemental zinc 3 times daily) ... Whether the increase in these enzyme levels in serum is indicative of pancreatic injury remains questionable.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 2:265
FDA Pregnancy Risk Category: A /CONTROLLED STUDIES SHOW NO RISK. Adequate, well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester of pregnancy./
US Natl Inst Health; DailyMed. Current Medication Information for Galzin - Zinc Acetate (April 2006). Available from, as of August 26, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1081
If this drug is used during pregnancy, the possibility of fetal harm appears remote. Because studies cannot rule out the possibility of harm, however, zinc acetate should be used during pregnancy only if clearly needed. While zinc acetate should be used during pregnancy only if clearly needed, copper toxicosis can develop during pregnancy if anti-copper therapy is stopped.
US Natl Inst Health; DailyMed. Current Medication Information for Galzin - Zinc Acetate (April 2006). Available from, as of August 26, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1081
For more Drug Warnings (Complete) data for ZINC ACETATE (7 total), please visit the HSDB record page.
Zinc can be used for the treatment and prevention of zinc deficiency/its consequences, including stunted growth and acute diarrhea in children, and slowed wound healing. It is also utilized for boosting the immune system, treating the common cold and recurrent ear infections, as well as preventing lower respiratory tract infections.
Zinc is involved in various aspects of cellular metabolism. It has been estimated that approximately 10% of human proteins may bind zinc, in addition to hundreds of proteins that transport and traffic zinc. It is required for the catalytic activity of more than 200 enzymes, and it plays a role in immune function wound healing, protein synthesis, DNA synthesis, and cell division. Zinc is an essential element for a proper sense of taste and smell and supports normal growth and development during pregnancy, childhood, and adolescence. It is thought to have antioxidant properties, which may be protective against accelerated aging and helps to speed up the healing process after an injury; however, studies differ as to its effectiveness. Zinc ions are effective antimicrobial agents even if administered in low concentrations. Studies on oral zinc for specific conditions shows the following evidence in various conditions: **Colds:** Evidence suggests that if zinc lozenges or syrup are taken within 24 hours after cold symptoms start, the supplement may shorten the length of colds. The use intranasal zinc has been associated with the loss of the sense of smell, in some cases long-term or permanently. **Wound healing:** Patients with skin ulcers and decreased levels of zinc may benefit from oral zinc supplements. **Diahrrea**: Oral zinc supplements can reduce the symptoms of diarrhea in children with low levels of zinc, especially in cases of malnutrition.
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AX - Various alimentary tract and metabolism products
A16AX05 - Zinc acetate
Absorption
Zinc is absorbed in the small intestine by a carrier-mediated mechanism. Under regular physiologic conditions, transport processes of uptake do not saturate. The exact amount of zinc absorbed is difficult to determine because zinc is secreted into the gut. Zinc administered in aqueous solutions to fasting subjects is absorbed quite efficiently (at a rate of 60-70%), however, absorption from solid diets is less efficient and varies greatly, dependent on zinc content and diet composition. Generally, 33% is considered to be the average zinc absorption in humans. More recent studies have determined different absorption rates for various populations based on their type of diet and phytate to zinc molar ratio. Zinc absorption is concentration dependent and increases linearly with dietary zinc up to a maximum rate. Additionally zinc status may influence zinc absorption. Zinc-deprived humans absorb this element with increased efficiency, whereas humans on a high-zinc diet show a reduced efficiency of absorption.
Route of Elimination
The excretion of zinc through gastrointestinal tract accounts for approximately one-half of all zinc eliminated from the body. Considerable amounts of zinc are secreted through both biliary and intestinal secretions, however most is reabsorbed. This is an important process in the regulation of zinc balance. Other routes of zinc excretion include both urine and surface losses (sloughed skin, hair, sweat). Zinc has been shown to induce intestinal metallothionein, which combines zinc and copper in the intestine and prevents their serosal surface transfer. Intestinal cells are sloughed with approximately a 6-day turnover, and the metallothionein-bound copper and zinc are lost in the stool and are thus not absorbed. Measurements in humans of endogenous intestinal zinc have primarily been made as fecal excretion; this suggests that the amounts excreted are responsive to zinc intake, absorbed zinc and physiologic need. In one study, elimination kinetics in rats showed that a small amount of ZnO nanoparticles was excreted via the urine, however, most of the nanoparticles were excreted via the feces.
Volume of Distribution
A pharmacokinetic study was done in rats to determine the distribution and other metabolic indexes of zinc in two particle sizes. It was found that zinc particles were mainly distributed to organs including the liver, lung, and kidney within 72 hours without any significant difference being found according to particle size or rat gender.
Clearance
In one study of healthy patients, the clearance of zinc was found to be 0.63 0.39 g/min.
Zinc salts are not equal in solubility, which is important in zinc absorption. The solubility of zinc salts is affected by gastric pH. Healthy subjects were given a single oral dose of 50 mg elemental zinc as the acetate ... under either high (pH > 5) or low (pH < 3) intragastric pH conditions. The mean plasma zinc area under the curve for zinc acetate at low pH (AL) /and/ ... at high pH (AH) ... were 524 /and/ 378 ... ug/hr/dL ...
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 2:266
Absorption /of zinc acetate/ by the GI tract is variable in animals and poor in humans. Accumulation occurred in the liver and pancreas. Some regulation of intake and output of zinc probably takes place in the intestines. In rats and mice, metallothionein, a low-molecular-mass cytoplasmic metalloprotein, takes considerable part in this process. Excreted predominantly with feces. Urinary excretion is negligible.
Sheftel, V.O.; Indirect Food Additives and Polymers. Migration and Toxicology. Lewis Publishers, Boca Raton, FL. 2000., p. 430
In a study with human volunteers, most of the zinc in a zinc acetate solution (0.1 mmol/L) administered by intestinal perfusion was absorbed from the jejunum, followed by the duodenum and the ileum (357, 230 or 84 nmol/ liter per min per 40 cm respectively). The absorption showed a linear increase at concentrations of 0.1- 1.8 mmol/ L.
WHO; Environ Health Criteria 221: Zinc p. 85 (2001)
The absorption of zinc from soluble zinc acetate, zinc sulfate ... and insoluble zinc oxide was compared in ten human volunteers who were dosed orally with 50 mg Zn in various forms separated by two weeks intervals. Bioavailability of zinc from the various forms was compared on the basis of plasma zinc levels and area under the plasma curve (AUC) analysis. Plasma peak levels were observed after about 2.5 hours for all forms, but maximal plasma Zn concentration amounted to 221 and 225 ug/dL for the acetate and the sulphate form while the peak plasma level for Zn from the oxide was only 159 ug/dL. When AUC values for the different zinc forms were compared, it appeared that the bioavailability of zinc oxide was about 60% of the bioavailability of the soluble forms. /Zinc salts/
European Chemicals Bureau; EU Risk Assessment Report- Zinc chloride, Vol.45 p.27 (2004). Available from, as of July 07, 2006: https://esis.jrc.ec.europa.eu/
Zinc is released from food as free ions during its digestion. These freed ions may then combine with endogenously secreted ligands before their transport into the enterocytes in the duodenum and jejunum.. Selected transport proteins may facilitate the passage of zinc across the cell membrane into the hepatic circulation. With high intake, zinc may also be absorbed through a passive paracellular route. The portal system carries absorbed zinc directly into the hepatic circulation, and then it is released into systemic circulation for delivery to various tissues. Although, serum zinc represents only 0.1% of the whole body zinc, the circulating zinc turns over rapidly to meet tissue needs.
The half-life of zinc in humans is approximately 280 days.
**Zinc has three primary biological roles**: _catalytic_, _structural_, and _regulatory_. The catalytic and structural role of zinc is well established, and there are various noteworthy reviews on these functions. For example, zinc is a structural constituent in numerous proteins, inclusive of growth factors, cytokines, receptors, enzymes, and transcription factors for different cellular signaling pathways. It is implicated in numerous cellular processes as a cofactor for approximately 3000 human proteins including enzymes, nuclear factors, and hormones. Zinc promotes resistance to epithelial apoptosis through cell protection (cytoprotection) against reactive oxygen species and bacterial toxins, likely through the antioxidant activity of the cysteine-rich metallothioneins. In HL-60 cells (promyelocytic leukemia cell line), zinc enhances the up-regulation of A20 mRNA, which, via TRAF pathway, decreases NF-kappaB activation, leading to decreased gene expression and generation of tumor necrosis factor-alpha (TNF-alpha), IL-1beta, and IL-8. There are several mechanisms of action of zinc on acute diarrhea. Various mechanisms are specific to the gastrointestinal system: zinc restores mucosal barrier integrity and enterocyte brush-border enzyme activity, it promotes the production of antibodies and circulating lymphocytes against intestinal pathogens, and has a direct effect on ion channels, acting as a potassium channel blocker of adenosine 3-5-cyclic monophosphate-mediated chlorine secretion. Cochrane researchers examined the evidence available up to 30 September 2016. Zinc deficiency in humans decreases the activity of serum _thymulin_ (a hormone of the thymus), which is necessary for the maturation of T-helper cells. T-helper 1 (Th(1)) cytokines are decreased but T-helper 2 (Th(2)) cytokines are not affected by zinc deficiency in humans. The change of _Th(1)_ to _Th(2)_ function leads to cell-mediated immune dysfunction. Because IL-2 production (Th(1) cytokine) is decreased, this causes decreased activity of natural-killer-cell (NK cell) and T cytolytic cells, normally involved in killing viruses, bacteria, and malignant cells. In humans, zinc deficiency may lead to the generation of new CD4+ T cells, produced in the thymus. In cell culture studies (HUT-78, a Th(0) human malignant lymphoblastoid cell line), as a result of zinc deficiency, nuclear factor-kappaB (NF-kappaB) activation, phosphorylation of IkappaB, and binding of NF-kappaB to DNA are decreased and this results in decreased Th(1) cytokine production. In another study, zinc supplementation in human subjects suppressed the gene expression and production of pro-inflammatory cytokines and decreased oxidative stress markers. In HL-60 cells (a human pro-myelocytic leukemia cell line), zinc deficiency increased the levels of TNF-alpha, IL-1beta, and IL-8 cytokines and mRNA. In such cells, zinc was found to induce A20, a zinc finger protein that inhibited NF-kappaB activation by the tumor necrosis factor receptor-associated factor pathway. This process decreased gene expression of pro-inflammatory cytokines and oxidative stress markers. The exact mechanism of zinc in acne treatment is poorly understood. However, zinc is considered to act directly on microbial inflammatory equilibrium and facilitate antibiotic absorption when used in combination with other agents. Topical zinc alone as well as in combination with other agents may be efficacious because of its anti-inflammatory activity and ability to reduce P. acnes bacteria by the inhibition of P. acnes lipases and free fatty acid levels.
The active moiety in zinc acetate is zinc cation. Regardless of the ligand, zinc blocks the intestinal absorption of copper from the diet and the reabsorption of endogenously secreted copper such as that from the saliva, gastric juice and bile. Zinc induces the production of metallothionein in the enterocyte, a protein that binds copper thereby preventing its serosal transfer into the blood. The bound copper is then lost in the stool following desquamation of the intestinal cells.
US Natl Inst Health; DailyMed. Current Medication Information for Galzin - Zinc Acetate (April 2006). Available from, as of August 26, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=1081
The mechanism of zinc's anticopper action is unique. It induces intestinal cell metallothionein, which binds copper and prevents its transfer into blood. As intestinal cells die and slough, the contained copper is eliminated in the stool. Thus, zinc prevents the intestinal absorption of copper.
Brewer GJ et al; Hepatology 31 (2): 364-70 (2000)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE
82
PharmaCompass offers a list of Zinc Acetate Dihydrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Zinc Acetate Dihydrate manufacturer or Zinc Acetate Dihydrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Zinc Acetate Dihydrate manufacturer or Zinc Acetate Dihydrate supplier.
PharmaCompass also assists you with knowing the Zinc Acetate Dihydrate API Price utilized in the formulation of products. Zinc Acetate Dihydrate API Price is not always fixed or binding as the Zinc Acetate Dihydrate Price is obtained through a variety of data sources. The Zinc Acetate Dihydrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Zinc Acetate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Zinc Acetate, including repackagers and relabelers. The FDA regulates Zinc Acetate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Zinc Acetate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Zinc Acetate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Zinc Acetate supplier is an individual or a company that provides Zinc Acetate active pharmaceutical ingredient (API) or Zinc Acetate finished formulations upon request. The Zinc Acetate suppliers may include Zinc Acetate API manufacturers, exporters, distributors and traders.
click here to find a list of Zinc Acetate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Zinc Acetate DMF (Drug Master File) is a document detailing the whole manufacturing process of Zinc Acetate active pharmaceutical ingredient (API) in detail. Different forms of Zinc Acetate DMFs exist exist since differing nations have different regulations, such as Zinc Acetate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Zinc Acetate DMF submitted to regulatory agencies in the US is known as a USDMF. Zinc Acetate USDMF includes data on Zinc Acetate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Zinc Acetate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Zinc Acetate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Zinc Acetate Drug Master File in Japan (Zinc Acetate JDMF) empowers Zinc Acetate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Zinc Acetate JDMF during the approval evaluation for pharmaceutical products. At the time of Zinc Acetate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Zinc Acetate suppliers with JDMF on PharmaCompass.
A Zinc Acetate CEP of the European Pharmacopoeia monograph is often referred to as a Zinc Acetate Certificate of Suitability (COS). The purpose of a Zinc Acetate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Zinc Acetate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Zinc Acetate to their clients by showing that a Zinc Acetate CEP has been issued for it. The manufacturer submits a Zinc Acetate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Zinc Acetate CEP holder for the record. Additionally, the data presented in the Zinc Acetate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Zinc Acetate DMF.
A Zinc Acetate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Zinc Acetate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Zinc Acetate suppliers with CEP (COS) on PharmaCompass.
A Zinc Acetate written confirmation (Zinc Acetate WC) is an official document issued by a regulatory agency to a Zinc Acetate manufacturer, verifying that the manufacturing facility of a Zinc Acetate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Zinc Acetate APIs or Zinc Acetate finished pharmaceutical products to another nation, regulatory agencies frequently require a Zinc Acetate WC (written confirmation) as part of the regulatory process.
click here to find a list of Zinc Acetate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Zinc Acetate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Zinc Acetate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Zinc Acetate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Zinc Acetate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Zinc Acetate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Zinc Acetate suppliers with NDC on PharmaCompass.
Zinc Acetate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Zinc Acetate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Zinc Acetate GMP manufacturer or Zinc Acetate GMP API supplier for your needs.
A Zinc Acetate CoA (Certificate of Analysis) is a formal document that attests to Zinc Acetate's compliance with Zinc Acetate specifications and serves as a tool for batch-level quality control.
Zinc Acetate CoA mostly includes findings from lab analyses of a specific batch. For each Zinc Acetate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Zinc Acetate may be tested according to a variety of international standards, such as European Pharmacopoeia (Zinc Acetate EP), Zinc Acetate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Zinc Acetate USP).
