
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
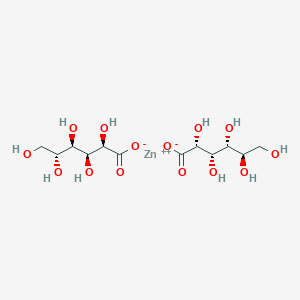

1. Boron Gluconate
2. D-gluconate
3. D-gluconic Acid
4. Dextronic Acid
5. Gluconate
6. Gluconic Acid
7. Gluconic Acid, (113)indium-labeled
8. Gluconic Acid, (14)c-labeled
9. Gluconic Acid, (159)dysprosium-labeled Salt
10. Gluconic Acid, (99)technecium (5+) Salt
11. Gluconic Acid, 1-(14)c-labeled
12. Gluconic Acid, 6-(14)c-labeled
13. Gluconic Acid, Aluminum (3:1) Salt
14. Gluconic Acid, Ammonium Salt
15. Gluconic Acid, Calcium Salt
16. Gluconic Acid, Cesium(+3) Salt
17. Gluconic Acid, Cobalt (2:1) Salt
18. Gluconic Acid, Copper Salt
19. Gluconic Acid, Fe(+2) Salt, Dihydrate
20. Gluconic Acid, Lanthanum(+3) Salt
21. Gluconic Acid, Magnesium (2:1) Salt
22. Gluconic Acid, Manganese (2:1) Salt
23. Gluconic Acid, Monolithium Salt
24. Gluconic Acid, Monopotassium Salt
25. Gluconic Acid, Monosodium Salt
26. Gluconic Acid, Potassium Salt
27. Gluconic Acid, Sodium Salt
28. Gluconic Acid, Strontium (2:1) Salt
29. Gluconic Acid, Tin(+2) Salt
30. Gluconic Acid, Zinc Salt
31. Lithium Gluconate
32. Magnerot
33. Magnesium Gluconate
34. Maltonic Acid
35. Manganese Gluconate
36. Pentahydroxycaproic Acid
37. Sodium Gluconate
1. 4468-02-4
2. Zincgluconate
3. Zincum Gluconicum
4. Gluconic Acid Zinc Complex
5. Zinc D-gluconate (1:2)
6. Bis(d-gluconato-o1,o2) Zinc
7. Gluconic Acid Zinc(ii) Salt
8. Zinc Gluconate (usp)
9. U6wsn5sq1z
10. Zinc, Bis(d-gluconato-kappao1,kappao2)-, (t-4)-
11. Gluconic Acid Zinc
12. Zinc(ii) Gluconate
13. Schembl21280
14. Zinc Gluconate [fcc]
15. Zinc Gluconate [hsdb]
16. Zinc Gluconate [inci]
17. Zinc Gluconate [vandf]
18. Zinc Gluconate [mart.]
19. Chembl3833377
20. Zinc Gluconate [who-dd]
21. Zincum Gluconicum [hpus]
22. Chebi:29708
23. Dtxsid20894125
24. Zinc Gluconate [green Book]
25. Zinc Gluconate [ep Impurity]
26. Zinc Gluconate [ep Monograph]
27. Akos015951235
28. Zinc Gluconate [usp Monograph]
29. Db11248
30. Gluconic Acid Zinc Complex [mi]
31. G0277
32. D02390
33. F71322
34. A826659
35. Q-201160
36. Q3822815
37. Zinc(ii) (2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanoate
38. Zinc (2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanoate;zinc Gluconate Hydrate
| Molecular Weight | 455.7 g/mol |
|---|---|
| Molecular Formula | C12H22O14Zn |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 8 |
| Exact Mass | 454.030097 g/mol |
| Monoisotopic Mass | 454.030097 g/mol |
| Topological Polar Surface Area | 283 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 165 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
MEDICATION (VET): CATTLE DERMATOMYCOSIS, CAUSED BY TRICHOPHYTON VERRUCOSUM (TRICHOPHYTOSIS) WAS TREATED BY ORAL ADMIN OF PREPN CONTAINING 20-70% ZINC GLUCONATE, 2-10% SULFUR, 0.2-1% VITAMIN A (106 IU), 2-6% METHIONINE, & INERT CARRIER SUCH AS CALCIUM CARBONATE OR FLOUR.
KOVAC L; CZECH PATENT 184924 09/15/78
DIETARY SUPPLEMENT & FOOD ADDITIVE; VITAMIN TABLETS
Sax, N.I. and R.J. Lewis, Sr. (eds.). Hawley's Condensed Chemical Dictionary. 11th ed. New York: Van Nostrand Reinhold Co., 1987., p. 1253
EXPTL USE: ZINC GLUCONATE ADDED TO SUBOPTIMAL DOSES OF ORTHO-GYNOL JELLY OR DELFEN CREAM IMPROVES VAGINAL CONTRACEPTIVE EFFICACY OF THESE PRODUCTS.
PMID:7214913 WILLIAMS WL; CONTRACEPTION 22 (6): 659-72 (1980)
EXPTL USE: TOXIC BLOOD LEAD LEVELS IN WORKERS DECR FROM 61.6 TO 46.0 UG/100 ML AFTER 24 WK TREATMENT WITH VITAMIN C & ZINC (2 G & 60 MG, DAILY). HEMOGLOBIN LEVELS INCR SIGNIFICANTLY & SERUM & WHOLE BLOOD COPPER LEVELS DECREASED FOLLOWING TREATMENT.
PAPAIOANNOU R ET AL; J ORTHOMOL PSYCHIATRY 7 (2): 94-106 (1978)
For more Therapeutic Uses (Complete) data for ZINC GLUCONATE (8 total), please visit the HSDB record page.
A potential role for zinc in retarding the progression of age-related macular degeneration has not been proven. Zinc salts have not been found to be beneficial in the treatment of acute intermittent porphyria. /Zinc supplements/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 3020
Zinc injection that contains benzyl alcohol as a preservative should not be used in newborn and immature infants. The use of benzyl alcohol in neonates has been associated with a fatal toxic syndrome consisting of metabolic acidosis and CNS, respiratory, circulatory, and renal function impairment. /Zinc supplements/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 3020
Zinc gluconate is mainly indicated in conditions like zinc deficiency, and can also be administered in adjunctive therapy as an alternative drug of choice in diarrhea.
Zinc is an important mineral found in almost every cell in the human body. It promotes the activity of about 100 enzymes. Zinc deficiency is often associated with an increased risk of infection. When they are used to treat the common cold, zinc supplements may interfere with rhinovirus cleavage or adhesion and may play a role in protecting plasma membranes from microbial toxins and complement.
A - Alimentary tract and metabolism
A12 - Mineral supplements
A12C - Other mineral supplements
A12CB - Zinc
A12CB02 - Zinc gluconate
Absorption
Please refer to DrugBank entry [DB01593]
Route of Elimination
Feces and urine
Volume of Distribution
Stored primarily in skeletal muscle and bone.
Clearance
Please refer to DrugBank entry [DB01593]
Please refer to DrugBank entry [DB01593]
280 days
Although the mechanism of action is not completely known, zinc supplementation may be used to increase immunity against viruses or may interfere with the replication of certain viruses, such as the human papillomavirus (HPV).
ABOUT THIS PAGE
38
PharmaCompass offers a list of Zinc(II) Gluconate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Zinc(II) Gluconate manufacturer or Zinc(II) Gluconate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Zinc(II) Gluconate manufacturer or Zinc(II) Gluconate supplier.
PharmaCompass also assists you with knowing the Zinc(II) Gluconate API Price utilized in the formulation of products. Zinc(II) Gluconate API Price is not always fixed or binding as the Zinc(II) Gluconate Price is obtained through a variety of data sources. The Zinc(II) Gluconate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Zinc(II) Gluconate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Zinc(II) Gluconate, including repackagers and relabelers. The FDA regulates Zinc(II) Gluconate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Zinc(II) Gluconate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Zinc(II) Gluconate supplier is an individual or a company that provides Zinc(II) Gluconate active pharmaceutical ingredient (API) or Zinc(II) Gluconate finished formulations upon request. The Zinc(II) Gluconate suppliers may include Zinc(II) Gluconate API manufacturers, exporters, distributors and traders.
Zinc(II) Gluconate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Zinc(II) Gluconate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Zinc(II) Gluconate GMP manufacturer or Zinc(II) Gluconate GMP API supplier for your needs.
A Zinc(II) Gluconate CoA (Certificate of Analysis) is a formal document that attests to Zinc(II) Gluconate's compliance with Zinc(II) Gluconate specifications and serves as a tool for batch-level quality control.
Zinc(II) Gluconate CoA mostly includes findings from lab analyses of a specific batch. For each Zinc(II) Gluconate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Zinc(II) Gluconate may be tested according to a variety of international standards, such as European Pharmacopoeia (Zinc(II) Gluconate EP), Zinc(II) Gluconate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Zinc(II) Gluconate USP).
