
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
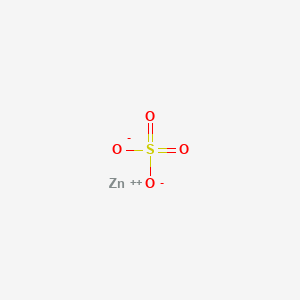

1. Sulfate, Zinc
2. Zinc Sulfate, Heptahydrate
3. Zincteral
1. Zinc Sulphate
2. 7733-02-0
3. Zinc Sulfate Anhydrous
4. Zincate
5. Zinc Sulfate (1:1)
6. Znso4
7. Sulfuric Acid, Zinc Salt (1:1)
8. Sulfate De Zinc
9. Zinc(ii) Sulfate
10. Zinc Sulphate Anhydrous
11. Zinc Sulfate, Anhydrous
12. Zinc Sulphate, Anhydrous
13. Zinc Sulfate (anhydrous)
14. Zinc Sulfate Hydrate
15. 0j6z13x3wo
16. Chebi:35176
17. Nsc-32677
18. Nsc-135806
19. Complexonat
20. Medizinc
21. Optised
22. Orazinc
23. Solvezinc
24. Zinklet
25. Neozin
26. Visine-ac
27. Zinci Sulfas
28. Prefrin-z
29. Zincum Sulfuricum
30. Zink-gro
31. Zinc Sulfate Solution
32. Zinc Vitriol (van)
33. Caswell No. 927
34. White Vitriol (van)
35. Zincsulphate
36. Salvazinc
37. Zinc;sulfate
38. Nu-z
39. Sulfuric Acid, Zinc Salt
40. Zinc-200
41. Sulfate De Zinc [french]
42. Ccris 3664
43. Sulfuric Acid Zinc Salt (van)
44. Hsdb 1063
45. Einecs 231-793-3
46. Nsc 32677
47. Epa Pesticide Chemical Code 089001
48. Nsc 135806
49. Unii-0j6z13x3wo
50. Ai3-03967
51. Sulfato De Cinc
52. Zinc(ii)sulfate
53. Sulfato De Zinco
54. Solfato Di Zinco
55. Zinc (as Sulfate)
56. Zinc(2+) Sulfate
57. Mfcd00011302
58. Zinc (as Sulphate)
59. Zinc Sulphate Hydrate
60. Zinci Sulfas Monohydrate
61. Zinc Suflate Monohydrate
62. Zinc Sulfate, Unspecified
63. Zinc Sulfate [mi]
64. Zinc Sulphate Nanoparticles
65. Ec 231-793-3
66. Zinc Sulfate [hsdb]
67. Zinc Sulfate, Unspecified Form
68. Chembl1200929
69. Dtxsid2040315
70. Akos025295737
71. Zinc Sulfate, 100mm Aqueous Solution
72. Db09322
73. Q204954
74. J-010404
| Molecular Weight | 161.4 g/mol |
|---|---|
| Molecular Formula | O4SZn |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 159.880871 g/mol |
| Monoisotopic Mass | 159.880871 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Mesh Heading: Astringent
National Library of Medicine, SIS; ChemIDplus Record for Zinc Sulfate. (7733-02-0). Available from, as of April 17, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Dietary supplement ingredient in use before October 15, 1994 (Mineral and trace element) /Date of the Dietary Supplement Health and Education Act of 1994/..
NNFA List of Dietary Supplement Ingredients In Use Before October 15, 1994. Available from, as of December 8, 2006: https://www.fda.gov/ohrms/dockets/dockets/05p0305/05p-0305-cr00001-03-NNFA-List-vol1.pdf
MEDICATION (VET): Zinc sulfate, USP, applied topically in 10% aqueous solution, is highly effective in treatment of foot rot in sheep.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 670
MEDICATION (VET): Useful antiseptic astringent for ophthalmic use as aqueous solution or ointment (0.25-0.5%). Spraying sheep with 0.25% solution after shearing reduced incidence of infected wounds. 1% solutions have been used on granular vaginitis lesions of cows, posthitis of rams, and make excellent detergent, odorless, antiseptic egg wash. ... As white lotion ... on bruised, inflamed, pruritic skin areas on cows and horses.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 669
For more Therapeutic Uses (Complete) data for ZINC SULFATE (26 total), please visit the HSDB record page.
... Zinc sulfate often is an effective /emetic/, but potential hemolytic and renal toxicity is too great to recommend use ... .
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 489
Thiazide diuretics have been found to increase urinary zinc excretion. /Zinc supplements/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Large doses of zinc may inhibit copper absorption in the intestine; zinc supplements should be taken at least 2 hours after the administration of copper supplements. /Zinc supplements/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Patients using zinc sulfate ophthalmic solutions should be advised to discontinue the drug and consult a physician if ocular pain or visual changes occur, they experience continued ocular redness or irritation, or the condition worsens or persists for more than 3 days.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 2761
For more Drug Warnings (Complete) data for ZINC SULFATE (15 total), please visit the HSDB record page.
This medication is a mineral used to treat or prevent low levels of zinc alone and together with oral rehydration therapy (ORT). It is also used as a topical astringent. Zinc Sulfate Injection, USP is indicated for use as a supplement to intravenous solutions given for TPN.
Zinc sulfate is a common zinc supplement in parenteral nutrition.
Zinc has been identified as a cofactor for over 70 different enzymes, including alkaline phosphatase, lactic dehydrogenase and both RNA and DNA polymerase. Zinc facilitates wound healing, helps maintain normal growth rates, normal skin hydration and the senses of taste and smell.
Astringents
Agents, usually topical, that cause the contraction of tissues for the control of bleeding or secretions. (See all compounds classified as Astringents.)
A - Alimentary tract and metabolism
A12 - Mineral supplements
A12C - Other mineral supplements
A12CB - Zinc
A12CB01 - Zinc sulfate
B - Blood and blood forming organs
B05 - Blood substitutes and perfusion solutions
B05X - I.v. solution additives
B05XA - Electrolyte solutions
B05XA18 - Zinc sulfate
Absorption
Approximately 20 to 30% of dietary zinc is absorbed, primarily from the duodenum and ileum. The amount absorbed is dependent on the bioavailability from food. Zinc is the most bioavailable from red meat and oysters. Phytates may impair absorption by chelation and formation of insoluble complexes at an alkaline pH. After absorption, zinc is bound in the intestine to the protein metallothionein. Endogenous zinc can be reabsorbed in the ileum and colon, creating an enteropancreatic circulation of zinc.
Route of Elimination
Primarily fecal (approximately 90%); to a lesser extent in the urine and in perspiration.
Volume of Distribution
After absorption zinc is bound to protein metallothionein in the intestines. Zinc is widely distributed throughout the body. It is primarily stored in RBCs, WBCs, muscles, bones, Skin, Kidneys, Liver, Pancreas, retina, and prostate.
The pharmacokinetics of zinc sulfate were compared with those of a new zinc pantothenate, in rabbits. Each salt was administered to rabbits at a dosage of 3.3 uCi of zinc-65/kg of body weight. The measured pharmacokinetics of the two compounds responded to a two compartment open model. The urinary elimination of the two salts was similar, as was their localization in the skin and fur, but Zn pantothenate was retained by the liver to a lesser extent than was zinc sulfate (ZnSO4).
PMID:6520772 Guillard O et al; J Pharm Sci 73 (11): 1642-3 (1984)
Absorption: 20% to 30%. Protein binding: 99%. Elimination: Through small bowel excretion.
Lelkin, J.B., Paloucek, F.P., Poisoning & Toxicology Compendium. LEXI-COMP Inc. & American Pharmaceutical Association, Hudson, OH 1998., p. 573
In women in different trimesters of pregnancy, the oral administration of 200 mg zinc sulfate per day resulted in an increase of serum zinc levels from 109.7 to 205.4 mg/dL. In the control group, serum zinc levels declined from 113.0 mg/dL in the first trimester to 83.8 in the third trimester.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 2:267
...An absorption half-life of 0.4 hr /was reported/ when 45 mg Zn2+ as zinc sulfate was administered once in gelatine capsules to 10 healthy young men. Serum concentrations were measured frequently during a total investigation time of 8 hours. A mean maximum concentration of 8.2 umol Zn2+/L serum was found after 2.3 hours (tmax). There is evidence of an enteral recirculation, the first rebound effect appeared after 1.4 hours during the absorption phase before tmax was reached, and exhibited mean reabsorption rates of 70% of the dose given. The subsequent ones (max. of 5) appeared at regular intervals of 1.2 hours with a decrease of the quantity reabsorbed.
European Chemicals Bureau; EU Risk Assessment Report- Zinc sulphate, Vol.46 p.31 (2004). Available from, as of June 29, 2006: https://esis.jrc.ec.europa.eu/
For more Absorption, Distribution and Excretion (Complete) data for ZINC SULFATE (14 total), please visit the HSDB record page.
3 hours
Half-life: 3 hours
Lelkin, J.B., Paloucek, F.P., Poisoning & Toxicology Compendium. LEXI-COMP Inc. & American Pharmaceutical Association, Hudson, OH 1998., p. 573
Zinc inhibits cAMP-induced, chloride-dependent fluid secretion by inhibiting basolateral potassium (K) channels, in in-vitro studies with rat ileum. This study has also shown the specificity of Zn to cAMP-activated K channels, because zinc did not block the calcium (Ca)-mediated K channels. As this study was not performed in Zn-deficient animals, it provides evidence that Zn is probably effective in the absence of Zn deficiency. Zinc also improves the absorption of water and electrolytes, improves regeneration of the intestinal epithelium, increases the levels of brush border enzymes, and enhances the immune response, allowing for a better clearance of the pathogens.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
34
PharmaCompass offers a list of Zinc Sulphate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Zinc Sulphate manufacturer or Zinc Sulphate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Zinc Sulphate manufacturer or Zinc Sulphate supplier.
PharmaCompass also assists you with knowing the Zinc Sulphate API Price utilized in the formulation of products. Zinc Sulphate API Price is not always fixed or binding as the Zinc Sulphate Price is obtained through a variety of data sources. The Zinc Sulphate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Zinc Sulphate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Zinc Sulphate, including repackagers and relabelers. The FDA regulates Zinc Sulphate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Zinc Sulphate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Zinc Sulphate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Zinc Sulphate supplier is an individual or a company that provides Zinc Sulphate active pharmaceutical ingredient (API) or Zinc Sulphate finished formulations upon request. The Zinc Sulphate suppliers may include Zinc Sulphate API manufacturers, exporters, distributors and traders.
click here to find a list of Zinc Sulphate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Zinc Sulphate DMF (Drug Master File) is a document detailing the whole manufacturing process of Zinc Sulphate active pharmaceutical ingredient (API) in detail. Different forms of Zinc Sulphate DMFs exist exist since differing nations have different regulations, such as Zinc Sulphate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Zinc Sulphate DMF submitted to regulatory agencies in the US is known as a USDMF. Zinc Sulphate USDMF includes data on Zinc Sulphate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Zinc Sulphate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Zinc Sulphate suppliers with USDMF on PharmaCompass.
Zinc Sulphate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Zinc Sulphate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Zinc Sulphate GMP manufacturer or Zinc Sulphate GMP API supplier for your needs.
A Zinc Sulphate CoA (Certificate of Analysis) is a formal document that attests to Zinc Sulphate's compliance with Zinc Sulphate specifications and serves as a tool for batch-level quality control.
Zinc Sulphate CoA mostly includes findings from lab analyses of a specific batch. For each Zinc Sulphate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Zinc Sulphate may be tested according to a variety of international standards, such as European Pharmacopoeia (Zinc Sulphate EP), Zinc Sulphate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Zinc Sulphate USP).
