

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
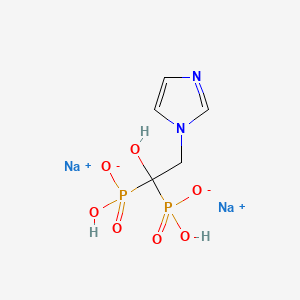

1. Zoledronic Acid Disodium
2. Zoledronate Disodium Anhydrous
3. 165800-07-7
4. 5csv5k1879
5. 131654-46-1
6. Unii-5csv5k1879
7. Dtxsid80157167
8. Akos015969312
9. J-010228
10. Q27261841
11. Phosphonic Acid, (1-hydroxy-2-(1h-imidazol-1-yl)ethylidene)bis-, Disodium Salt
12. Phosphonic Acid, P,p'-(1-hydroxy-2-(1h-imidazol-1-yl)ethylidene)bis-, Sodium Salt (1:2)
| Molecular Weight | 316.05 g/mol |
|---|---|
| Molecular Formula | C5H8N2Na2O7P2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 315.96021316 g/mol |
| Monoisotopic Mass | 315.96021316 g/mol |
| Topological Polar Surface Area | 159 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 322 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
ABOUT THIS PAGE
We have 1 companies offering zoledronic acid anhydrous
Get in contact with the supplier of your choice:


LOOKING FOR A SUPPLIER?
