
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
Canada

0
South Africa

0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
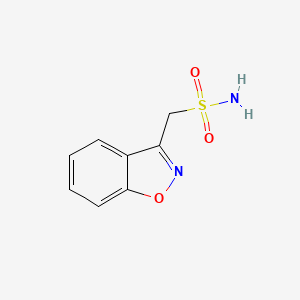

1. 3 Sulfamoylmethyl 1,2 Benzisoxazole
2. 3-sulfamoylmethyl-1,2-benzisoxazole
3. Ad 810
4. Ad-810
5. Ad810
6. Ci 912
7. Ci-912
8. Ci912
9. Zonegran
10. Zonisamide Monosodium
1. 68291-97-4
2. Zonegran
3. 1,2-benzisoxazole-3-methanesulfonamide
4. 1,2-benzoxazol-3-ylmethanesulfonamide
5. Exceglan
6. Excegram
7. Excegran
8. Zonisamidum [latin]
9. Zonisamida [spanish]
10. Ad-810
11. Zonisamida
12. Zonisamidum
13. Ci-912
14. 3-(sulfamoylmethyl)-1,2-benzisoxazole
15. Benzo[d]isoxazol-3-ylmethanesulfonamide
16. Ad 810
17. Ci 912
18. Pd 110843
19. Pd-110843
20. 1-(1,2-benzoxazol-3-yl)methanesulfonamide
21. Spr_2
22. Benzo[d]isoxazol-3-yl-methanesulfonamide
23. 1-(1,2-benzisoxazol-3-yl)methanesulfonamide
24. Ncgc00159319-02
25. Ncgc00159319-04
26. 459384h98v
27. Dsstox_cid_26023
28. Dsstox_rid_81296
29. Dsstox_gsid_46023
30. Tremode
31. Trerief
32. Excegran (tn)
33. Smr000596519
34. Zonisamide (zns)
35. Cas-68291-97-4
36. Hsdb 7293
37. Sr-01000837537
38. Brn 1077076
39. Zonisamide, Zns
40. Ad-810n
41. Zonisamide, 1
42. Unii-459384h98v
43. Zonisamide Solution
44. Mfcd00865316
45. Zonisamide [usan:usp:inn:ban:jan]
46. Zonisamide [mi]
47. Zonisamide [inn]
48. Zonisamide [jan]
49. Zonisamide [hsdb]
50. Zonisamide [usan]
51. Zonisamide [vandf]
52. E-2090
53. Zonisamide [mart.]
54. Zonisamide [usp-rs]
55. Zonisamide [who-dd]
56. Bidd:pxr0183
57. Schembl35458
58. Mls001195632
59. Mls001306491
60. Bidd:gt0708
61. Zonisamide [ema Epar]
62. Gtpl7047
63. Zinc4321
64. Zonisamide (jp17/usp/inn)
65. Dtxsid9046023
66. Zonisamide [orange Book]
67. Bdbm10888
68. Hms2089o07
69. Hms2235l13
70. Hms3259n09
71. Hms3269f21
72. Hms3413l09
73. Hms3657e03
74. Hms3677l09
75. Hms3714e19
76. Hms3742m15
77. Hms3884i17
78. Zonisamide [usp Monograph]
79. Zonisamide 1.0 Mg/ml In Methanol
80. Bcp28480
81. Hy-b0124
82. Tox21_111569
83. Bbl010040
84. S1445
85. Stk711131
86. Akos001312269
87. Tox21_111569_1
88. Ac-1413
89. Ccg-220669
90. Cs-1888
91. Db00909
92. Ks-1142
93. Nc00637
94. 1,2-benzisoxazol-3-ylmethanesulfonamide
95. (1,2-benzoxazol-3-yl)methanesulfonamide
96. Ncgc00159319-03
97. Bz164593
98. Ft-0601524
99. Ft-0675931
100. Sw199135-2
101. Z0026
102. En300-50288
103. C07504
104. C76163
105. D00538
106. Ab00876297-09
107. Ab00876297-10
108. Ab00876297_11
109. Zonisamide Pound>>ci-912 Pound>>pd 110843
110. 291z974
111. A836085
112. Q219957
113. Q-201948
114. Sr-01000837537-2
115. Sr-01000837537-3
116. Sr-01000837537-8
117. Brd-k48300629-001-03-8
118. Z223042524
119. Zonisamide Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
120. Zon
| Molecular Weight | 212.23 g/mol |
|---|---|
| Molecular Formula | C8H8N2O3S |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 212.02556330 g/mol |
| Monoisotopic Mass | 212.02556330 g/mol |
| Topological Polar Surface Area | 94.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 298 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Zonegran |
| PubMed Health | Zonisamide (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | ZONEGRAN (zonisamide) is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The empirical formula is C8H8N2O3S with a mo... |
| Active Ingredient | Zonisamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Eisai |
| 2 of 4 | |
|---|---|
| Drug Name | Zonisamide |
| PubMed Health | Zonisamide (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The molecular formula is C8H8N2O3S with a molecular weigh... |
| Active Ingredient | Zonisamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Wockhardt; Ani Pharms; Sun Pharm Inds (in); Apotex; Banner Pharmacaps; Invagen Pharms; Glenmark Generics; Zydus Pharms Usa; Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Zonegran |
| PubMed Health | Zonisamide (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | ZONEGRAN (zonisamide) is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The empirical formula is C8H8N2O3S with a mo... |
| Active Ingredient | Zonisamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Eisai |
| 4 of 4 | |
|---|---|
| Drug Name | Zonisamide |
| PubMed Health | Zonisamide (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | Zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The molecular formula is C8H8N2O3S with a molecular weigh... |
| Active Ingredient | Zonisamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Wockhardt; Ani Pharms; Sun Pharm Inds (in); Apotex; Banner Pharmacaps; Invagen Pharms; Glenmark Generics; Zydus Pharms Usa; Mylan |
Anticonvulsant
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1817
Zonisamide is indicated for adjunctive therapy use in the treatment of partial seizures in adults with epilepsy. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2903
Zonisamide (ZNS)-induced behavior disorders are reported in a 1-year-old girl and a 3-year-old boy. Both patients, who had no previous developmental or mental problems, displayed secondarily generalized motor seizures. Serum concentrations of ZNS were not high, 8.8 and 12.3 micrograms/ml (effective range 10-30 micrograms/ml) respectively. Although many cases of ZNS-related psychotic reactions and/or behavior disorders have been reported, all affected patients had complex partial seizures (CPS) and had received combination therapy with phenytoin (PHT). Thus, whether the disorders were induced only by ZNS, by an interaction between ZNS and PHT, or by CPS could not be determined. In the children reported, however, ZNS clearly induced behavior disorders at plasma ZNS levels within or even below the therapeutic range.
PMID:8156965 Kimura S; Epilepsia 35 (2): 403-5 (1994)
Oligohidrosis and Hyperthermia in Pediatric Patients: Oligohidrosis, sometimes resulting in heat stroke and hospitalization, is seen in association with zonisamide in pediatric patients. ... Decreased sweating and an elevation in body temperature above normal characterized these cases. Many cases were reported after exposure to elevated environmental temperatures. Heat stroke, requiring hospitalization, was diagnosed in some cases. There have been no reported deaths. Pediatric patients appear to be at an increased risk for zonisamide-associated oligohidrosis and hyperthermia. Patients, especially pediatric patients, treated with Zonegran should be monitored closely for evidence of decreased sweating and increased body temperature, especially in warm or hot weather. Caution should be used when zonisamide is prescribed with other drugs that predispose patients to heat-related disorders; these drugs include, but are not limited to, carbonic anhydrase inhibitors and drugs with anticholinergic activity.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 1232
Fatalities resulting from severe reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have occurred following use of zonisamide. Use of sulfonamides also rarely has caused fatalities resulting from fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias, regardless of the route of administration. Zonisamide should be discontinued immediately if signs or symptoms of hypersensitivity occur. Discontinuance of zonisamide should be considered whenever a patient receiving zonisamide develops unexplained rash; if the drug is not discontinued, the patient should be observed frequently.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2159
During the premarketing development of zonisamide, 9 sudden and unexplained deaths were reported among a cohort of 991 patients with epilepsy receiving adjunctive therapy with the drug (7.7 deaths per 1000 patient-years). Although the rate of these deaths exceeds that expected to occur in a healthy (nonepileptic) population, this rate was similar to that occurring in patients with refractory epilepsy not receiving zonisamide.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2159
For more Drug Warnings (Complete) data for ZONISAMIDE (22 total), please visit the HSDB record page.
For use as adjunctive treatment of partial seizures in adults with epilepsy.
FDA Label
Zonegran is indicated as:
- monotherapy in the treatment of partial seizures, with or without secondary generalisation, in adults with newly diagnosed epilepsy;
- adjunctive therapy in the treatment of partial seizures, with or without secondary generalisation, in adults, adolescents, and children aged six years and above.
Zonisamide is a sulfonamide and therefore unrelated to other seizure medications. The mechanism is not know but it may block sodium and calcium channels. Blocking of these channels may prevent neuronal hypersynchronization. Sonisamide has also been found to potentiate dopaminergic and serotonergic neurotransmission but does not appear to potentiate syanptic activity by GABA (gamma amino butyric acid).
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AX15
N03AX15
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX15 - Zonisamide
Absorption
The absorption is rapid with a time to peak concentration of 2.8-3.9 hours. Food has not effect on bioavailability.
Route of Elimination
Zonisamide is excreted primarily in urine as parent drug and as the glucuronide of a metabolite.
Volume of Distribution
1.45 L/kg
Clearance
0.30 - 0.35 mL/min/kg [patients not receiving enzyme-inducing antiepilepsy drugs (AEDs)]
0.35 - 0.5 mL/min/kg [Concomitant administration of phenytoin and carbamazepine]
Elimination: Renal: 62%, Fecal: 3%. Plasma clearance of zonisamide is approximately 0.30 to 0.35 mL/min/kg in patients not receiving concomitant therapy with enzyme-inducing anticonvulsants. Zonisamide clearance is increased to 0.5 mL/min/kg in patients concurrently receiving enzyme-inducing anticonvulsant medications.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2904
In patients with creatinine clearance <20 mL/min, the area under the concentration-time curve (AUC) for zonisamide is increased by 35%.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2904
Zonisamide is distributed to breast milk, cerebrospinal fluid, and erythrocytes. Concentration in erythrocytes is approximately 8 times higher than in plasma and the milk-to-plasma ratio is 0.93. The concentration of zonisamide in cerebrospinal fluid is approximately 76% of the concentration found in plasma.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2904
Following a 200-400 mg oral zonisamide dose, peak plasma concentrations (range: 2-5 ug/mL) in normal volunteers occur within 2-6 hours. In the presence of food, the time to maximum concentration is delayed, occurring at 4-6 hours, but food has no effect on the bioavailability of zonisamide. Zonisamide extensively binds to erythrocytes, resulting in an eight-fold higher concentration of zonisamide in red blood cells (RBC) than in plasma. The pharmacokinetics of zonisamide are dose proportional in the range of 200-400 mg, but the Cmax and AUC increase disproportionately at 800 mg, perhaps due to saturable binding of zonisamide to RBC. Once a stable dose is reached, steady state is achieved within 14 days. ...The apparent volume of distribution (V/F) of zonisamide is about 1.45 L/kg following a 400 mg oral dose. Zonisamide, at concentrations of 1.0-7.0 ug/mL, is approximately 40% bound to human plasma proteins. Protein binding of zonisamide is unaffected in the presence of therapeutic concentrations of phenytoin, phenobarbital or carbamazepine.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 1231
Zonisamide is excreted primarily in urine as parent drug and as the glucuronide of a metabolite. ... Of the excreted dose, 35% was recovered as zonisamide, 15% as N-acetyl zonisamide, and 50% as the glucuronide of 2-sulfamoylacetyl phenol (SMAP)
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 1232
Primarily hepatic through cytochrome P450 isoenzyme 3A4 (CYP3A4). Undergoes acetylation and reduction, forming N-acetyl zonisamide, and the open-ring metabolite 2–sulfamoylacetyl phenol, respectively.
... Zonisamide is excreted primarily in urine as parent drug and as the glucuronide of a metabolite. ... Zonisamide undergoes acetylation to form N-acetyl zonisamide and reduction to form the open ring metabolite, 2-sulfamoylacetyl phenol (SMAP). Of the excreted dose, 35% was recovered as zonisamide, 15% as N-acetyl zonisamide, and 50% as the glucuronide of SMAP. Reduction of zonisamide to SMAP is mediated by cytochrome p450 isozyme 3A4 (CYP3A4). Zonisamide does not induce its own metabolism.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 1232
Zonisamide undergoes acetylation to form N-acetyl zonisamide and reduction to form the open ring metabolite, 2-sulfamoylacetyl phenol (SMAP). ... Reduction of zonisamide to SMAP is mediated by cytochrome P450 isozyme 3A4 (CYP3A4). Zonisamide does not induce its own metabolism.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 1231
63 hours
Elimination /half-life/ in plasma: 63 hours; Elimination /half-life/ in erythrocytes: 105 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2904
Zonisamide binds to sodium channels and voltage sensitive calcium channels, which suppresses neuronal depolarization and hypersynchronization. Zonisamide also inhibits carbonic anhydrase to a weaker extent, but such an effect is not thought to contribute substantially to the drug's anticonvulsant activity.
The exact method by which zonisamide exerts its anticonvulsant effect is unknown. Some in vitro studies suggest a blockade of sodium channels, with consequent stabilization of neuronal membranes and suppression of neuronal hypersynchronization, whereas other in vitro studies have shown zonisamide to suppress synaptically-driven electrical activity without affecting postsynaptic GABA or glutamate responses. It appears then, that zonisamide dose not potentiate the synaptic activity of GABA. Zonisamide also serves as a weak inhibitor of carbonic anhydrase.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2903
Epileptiform discharges and behavioral seizures may be the consequences of excess excitation associated with the neurotransmitter glutamate, or from inadequate inhibitory effects associated with gamma-aminobutyric acid (GABA). Synaptic effects of these neurotransmitters are terminated by the action of transporter proteins that remove amino acids from the synaptic cleft. Excitation initiated by the synaptic release of glutamate is attenuated by the action of glial transporters glutamate-aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1), and the neuronal transporter excitatory amino-acid carrier-1 (EAAC-1). GABA is removed from synaptic regions by the action of the transporters proteins GABA transporter-1 (GAT-1) and GABA transporter-3 (GAT-3). Albino rats with chronic, spontaneous recurrent seizures induced by the amygdalar injection of eCl3 were treated for 14 days with zonisamide (ZNS) (40 mg/kg, ip). Control animals underwent saline injection into the same amygdalar regions. Treatment control for both groups of intracerebrally injected animals was ip injection of equal volumes of saline. Western blotting was used to measure the quantity of glutamate and GABA transporters in hippocampus and frontal cortex. ZNS caused increase in the quantity of EAAC-1 protein in hippocampus and cortex and down regulation of the GABA transporter GAT-1. These changes occurred in both experimental and ZNS treated control animals. These data show that the molecular effect of ZNS, with up-regulation of EAAC-1 and decreased production of GABA transporters, should result in increased tissue and synaptic concentrations of GABA.
PMID:12941455 Ueda Y et al; Brain Res Mol Brain Res 116 (1-2): 1-6 (2003)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
83
PharmaCompass offers a list of Zonisamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Zonisamide manufacturer or Zonisamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Zonisamide manufacturer or Zonisamide supplier.
PharmaCompass also assists you with knowing the Zonisamide API Price utilized in the formulation of products. Zonisamide API Price is not always fixed or binding as the Zonisamide Price is obtained through a variety of data sources. The Zonisamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Zonisamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Zonisamide, including repackagers and relabelers. The FDA regulates Zonisamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Zonisamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Zonisamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Zonisamide supplier is an individual or a company that provides Zonisamide active pharmaceutical ingredient (API) or Zonisamide finished formulations upon request. The Zonisamide suppliers may include Zonisamide API manufacturers, exporters, distributors and traders.
click here to find a list of Zonisamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Zonisamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Zonisamide active pharmaceutical ingredient (API) in detail. Different forms of Zonisamide DMFs exist exist since differing nations have different regulations, such as Zonisamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Zonisamide DMF submitted to regulatory agencies in the US is known as a USDMF. Zonisamide USDMF includes data on Zonisamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Zonisamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Zonisamide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Zonisamide Drug Master File in Japan (Zonisamide JDMF) empowers Zonisamide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Zonisamide JDMF during the approval evaluation for pharmaceutical products. At the time of Zonisamide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Zonisamide suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Zonisamide Drug Master File in Korea (Zonisamide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Zonisamide. The MFDS reviews the Zonisamide KDMF as part of the drug registration process and uses the information provided in the Zonisamide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Zonisamide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Zonisamide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Zonisamide suppliers with KDMF on PharmaCompass.
A Zonisamide written confirmation (Zonisamide WC) is an official document issued by a regulatory agency to a Zonisamide manufacturer, verifying that the manufacturing facility of a Zonisamide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Zonisamide APIs or Zonisamide finished pharmaceutical products to another nation, regulatory agencies frequently require a Zonisamide WC (written confirmation) as part of the regulatory process.
click here to find a list of Zonisamide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Zonisamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Zonisamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Zonisamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Zonisamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Zonisamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Zonisamide suppliers with NDC on PharmaCompass.
Zonisamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Zonisamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Zonisamide GMP manufacturer or Zonisamide GMP API supplier for your needs.
A Zonisamide CoA (Certificate of Analysis) is a formal document that attests to Zonisamide's compliance with Zonisamide specifications and serves as a tool for batch-level quality control.
Zonisamide CoA mostly includes findings from lab analyses of a specific batch. For each Zonisamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Zonisamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Zonisamide EP), Zonisamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Zonisamide USP).
