09 Apr 2025
// REUTERS
08 Apr 2025
// INDPHARMAPOST
03 Apr 2025
// REUTERS
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About
Product(s) under patent(s) are offered only for R&D purposes U/S 107A of the Patent Act and not for commercial sale
Industry Trade Show
Booth #5N-12
09-11 April, 2025
Industry Trade Show
Attending
22-24 April, 2025
CPhI North America CPhI North America
Industry Trade Show
Attending
20-22 May, 2025
CONTACT DETAILS




Events
Webinars & Exhibitions
Industry Trade Show
Booth #5N-12
09-11 April, 2025
Industry Trade Show
Attending
22-24 April, 2025
CPhI North America CPhI North America
Industry Trade Show
Attending
20-22 May, 2025
VLOG #PharmaReel
CORPORATE CONTENT #SupplierSpotlight
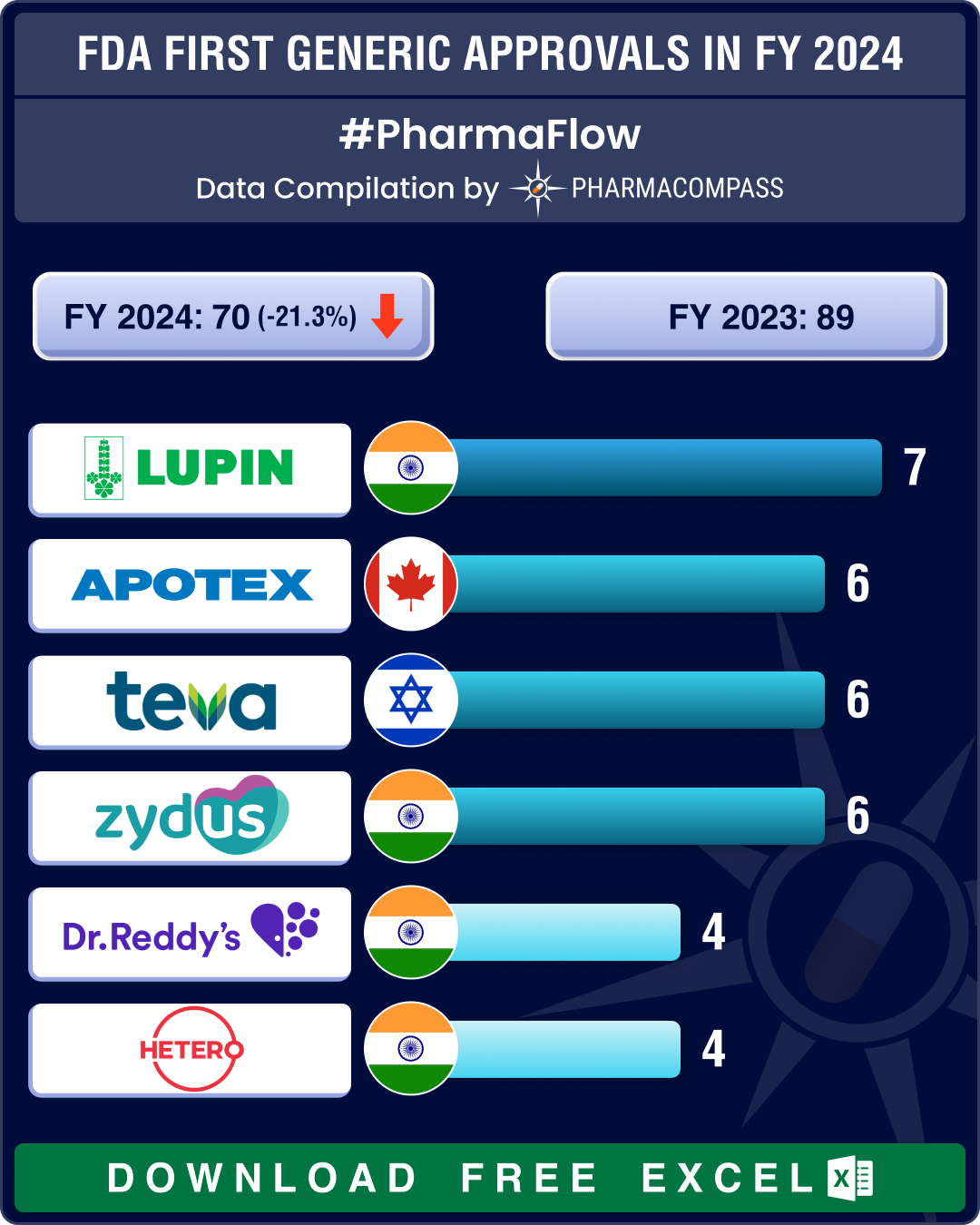
https://www.pharmacompass.com/radio-compass-blog/fda-s-first-generic-approvals-slump-21-in-2024-novartis-top-seller-entresto-cancer-blockbuster-tasigna-lead-2024-patent-cliff
https://www.pharmacompass.com/radio-compass-blog/fda-okays-50-new-drugs-in-2024-bms-cobenfy-lilly-s-kisunla-lead-pack-of-breakthrough-therapies
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-bora-polpharma-make-acquisitions-evonik-euroapi-porton-announce-technological-expansions
https://www.pharmacompass.com/radio-compass-blog/chinese-fda-registered-generic-facilities-gain-steam-india-maintains-lead-with-396-facilities
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-novo-s-parent-buys-catalent-for-us-16-5-bn-fujifilm-merck-kgaa-axplora-lonza-expand-capabilities
https://www.pharmacompass.com/radio-compass-blog/fda-approves-record-eight-biosimilars-in-h1-2024-okays-first-interchangeable-biosimilars-for-eylea
https://www.pharmacompass.com/radio-compass-blog/dmf-submissions-from-china-jump-42-as-india-continues-to-top-list-in-q1-2024

09 Apr 2025
// REUTERS
https://www.reuters.com/business/healthcare-pharmaceuticals/indian-pharma-stocks-decline-after-trump-again-threatens-tariffs-2025-04-09/https://www.reuters.com/business/healthcare-pharmaceuticals/indian-pharma-stocks-decline-after-trump-again-threatens-tariffs-2025-04-09/

08 Apr 2025
// INDPHARMAPOST
https://www.indianpharmapost.com/news/dr-reddys-laboratories-gets-rs-2395-crore-show-cause-notice-from-it-authority-16983

03 Apr 2025
// REUTERS
https://www.reuters.com/world/india/indian-pharma-stocks-defy-market-slump-us-tariffs-exemption-2025-04-03/

28 Mar 2025
// PRESS RELEASE
https://www.drreddys.com/cms/cms/sites/default/files/2025-03/DRL-%20Bio-Thera-%20press%20release-%2026.03.2025-%20final.pdf

19 Mar 2025
// PRESS RELEASE
https://investors.alvotech.com/news-releases/news-release-details/alvotech-and-dr-reddys-announce-fda-acceptance-biologic-license

16 Mar 2025
// ECONOMICTIMES
https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/big-pharma-mergers-unlikely-in-near-future-dr-reddys-md-gv-prasad/articleshow/119083888.cms
Excipients
Inspections and registrations
ABOUT THIS PAGE
Dr. Reddy\'s Laboratories is a supplier offers 279 products (APIs, Excipients or Intermediates).
Find a price of Atorvastatin bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Capecitabine bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Cetirizine Dihydrochloride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Clopidogrel bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Fexofenadine Hydrochloride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Finasteride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Gemcitabine bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Levetiracetam bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Montelukast Sodium bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Nizatidine bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Ondansetron Hydrochloride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Quetiapine Hemifumarate bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Rabeprazole Sodium bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Risperidone bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Rivaroxaban bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Valsartan bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Amlodipine Besylate bulk with DMF, CEP, JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Atorvastatin bulk with DMF, CEP, JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Azacitidine bulk with DMF, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Dasatinib bulk with DMF, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Desloratadine bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Esomeprazole Magnesium bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Famotidine bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Glimepiride bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Ketorolac Trometamol bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Lenalidomide bulk with DMF, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Levofloxacin Hemihydrate bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Losartan Potassium bulk with DMF, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Naproxen bulk with DMF, CEP, JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Naratriptan Hydrochloride bulk with DMF, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Omeprazole bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Omeprazole Magnesium bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Pantoprazole Sodium bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Pioglitazone Hydrochloride bulk with DMF, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Rabeprazole Sodium bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Raloxifene Hydrochloride bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Rivastigmine Tartrate bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Sitagliptin Phosphate bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Sugammadex Sodium bulk with DMF, JDMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Terbinafine Hydrochloride bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Ticagrelor bulk with DMF, CEP, WC offered by Dr. Reddy\'s Laboratories
Find a price of Abiraterone Acetate bulk with DMF, JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Apalutamide bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Apixaban bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Apremilast bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Atomoxetin Hydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Atorvastatin bulk with DMF, JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Bendamustine Hydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Bortezomib bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Cabazitaxel bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Carfilzomib bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Cinacalcet Hydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Dabigatran Etexilate Mesylate bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Dapagliflozin bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Dapagliflozin Propanediol Monohydrate bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Decitabine bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Dutasteride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Edaravone bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Elagolix Sodium bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Enzalutamide bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Eslicarbazepine Acetate bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Eszopiclone bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Fondaparinux Sodium bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Gatifloxacin bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Glatiramer Acetate bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Granisetron bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Iron Sucrose bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Lenvatinib Mesylate bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Levocetirizine Dihydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Linagliptin bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Lomustine bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Losartan Potassium bulk with DMF, JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Lurasidone Hydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Metoprolol Succinate bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Mirabegron bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Moxifloxacin Hydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Naproxen bulk with DMF, CEP offered by Dr. Reddy\'s Laboratories
Find a price of Naproxen Sodium bulk with DMF, CEP offered by Dr. Reddy\'s Laboratories
Find a price of Nilotinib bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Omeprazole Magnesium bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Ondansetron bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Palonosetron bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Pantoprazole Sodium bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Pemetrexed Disodium bulk with DMF, CEP offered by Dr. Reddy\'s Laboratories
Find a price of Permethrin bulk with DMF, CEP offered by Dr. Reddy\'s Laboratories
Find a price of Pomalidomide bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Posaconazole bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Pregabalin bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Ramipril bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Ranolazine bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Roxadustat bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Sacubitril-Valsartan bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Sitagliptin Hydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Sumatriptan bulk with DMF, CEP offered by Dr. Reddy\'s Laboratories
Find a price of Tizanidine HCl bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Ziprasidone Hydrochloride bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Zoledronic Acid bulk with DMF, WC offered by Dr. Reddy\'s Laboratories
Find a price of Abacavir bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Agomelatine bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Amtolmetin Guacil bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Apalutamide bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Apremilast bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Asenapine Maleate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Atomoxetin Hydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Atorvastatin bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Bempedoic Acid bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Benztropine bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Cabozantinib bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Capecitabine bulk with CEP offered by Dr. Reddy\'s Laboratories
Find a price of Cetirizine Dihydrochloride bulk with CEP offered by Dr. Reddy\'s Laboratories
Find a price of Dabigatran Etexilate Mesylate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Dasatinib bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Decitabine bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Divalproex Sodium bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Donepezil bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Doxazosin Mesylate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Duloxetine Hydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Eliglustat bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Empagliflozin bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Eribulin bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Escitalopram Oxalate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Esomeprazole Magnesium bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Ezetimibe bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Febuxostat bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Ferric Carboxymaltose bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Fexofenadine Hydrochloride bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Fingolimod Hydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Fluconazole bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Fosaprepitant bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Fosphenytoin Sodium bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Ibandronate Sodium bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Irbesartan bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Ketorolac Trometamol bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Lacidipine bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Lanreotide Acetate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Lansoprazole bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Lenalidomide bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Letrozole bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Levocetirizine Dihydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Lifitegrast bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Lubiprostone bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Lumateperone Tosylate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Memantine Hydrochloride bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Metaraminol Bitartrate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Metoprolol Succinate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Midostaurin bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Mirabegron bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Naproxen bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Nilotinib bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Olaparib bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Omeprazole bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Ondansetron Hydrochloride bulk with JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Oxaprozin bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Palbociclib bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Pazopanib Hydrochloride bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Pemetrexed Ditromethamine bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Plerixafor bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Pregabalin bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Rabeprazole Sodium bulk with CEP offered by Dr. Reddy\'s Laboratories
Find a price of Rasagiline Mesylate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Rasagiline Tartrate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Relugolix bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Ripretinib bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Roxadustat bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Sacubitril Sodium bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Sertraline Hydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Sildenafil Citrate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Sitagliptin Hydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Sitagliptin Phosphate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Solifenacin Succinate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Sumatriptan bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Tafamidis Meglumine bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Testosterone bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Tofacitinib Citrate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Tolterodine Tartrate bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Travoprost bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Treprostinil Sodium bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Valganciclovir Hydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Valsartan bulk with JDMF offered by Dr. Reddy\'s Laboratories
Find a price of Varenicline Tartrate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Venetoclax bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Vilazodone Hydrochloride bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Vonoprazan Fumarate bulk with DMF offered by Dr. Reddy\'s Laboratories
Find a price of Voriconazole bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Zafirlukast bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Zoledronic Acid bulk with WC offered by Dr. Reddy\'s Laboratories
Find a price of Abacavir Sulfate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Acalabrutinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Adagrasib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Alendronate Sodium bulk offered by Dr. Reddy\'s Laboratories
Find a price of Amlodipine Besylate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Anastrozole bulk offered by Dr. Reddy\'s Laboratories
Find a price of Apixaban bulk offered by Dr. Reddy\'s Laboratories
Find a price of Aprepitant bulk offered by Dr. Reddy\'s Laboratories
Find a price of Atorvastatin bulk offered by Dr. Reddy\'s Laboratories
Find a price of Bazedoxifene Acetate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Belumosudil bulk offered by Dr. Reddy\'s Laboratories
Find a price of Bivalirudin bulk offered by Dr. Reddy\'s Laboratories
Find a price of Brigatinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Carvedilol bulk offered by Dr. Reddy\'s Laboratories
Find a price of CAS 103639-04-9 bulk offered by Dr. Reddy\'s Laboratories
Find a price of CAS 654671-77-9 bulk offered by Dr. Reddy\'s Laboratories
Find a price of Cetirizine bulk offered by Dr. Reddy\'s Laboratories
Find a price of Cetirizine Dihydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ciprofloxacin Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Citalopram Hydrobromide bulk offered by Dr. Reddy\'s Laboratories
Find a price of Clopidogrel bulk offered by Dr. Reddy\'s Laboratories
Find a price of Dapagliflozin Propanediol Monohydrate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Darolutamide bulk offered by Dr. Reddy\'s Laboratories
Find a price of Deucravacitinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Dexlansoprazole bulk offered by Dr. Reddy\'s Laboratories
Find a price of Dimethyl Fumarate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Diroximel fumarate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Dutasteride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Edoxaban Tosylate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Empagliflozin bulk offered by Dr. Reddy\'s Laboratories
Find a price of Enalapril Maleate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Enalaprilat bulk offered by Dr. Reddy\'s Laboratories
Find a price of Etrasimod bulk offered by Dr. Reddy\'s Laboratories
Find a price of Everolimus bulk offered by Dr. Reddy\'s Laboratories
Find a price of Fezolinetant bulk offered by Dr. Reddy\'s Laboratories
Find a price of Finerenone bulk offered by Dr. Reddy\'s Laboratories
Find a price of Fruquintinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Galantamine Hydrobromide bulk offered by Dr. Reddy\'s Laboratories
Find a price of Gemcitabine bulk offered by Dr. Reddy\'s Laboratories
Find a price of Granisetron Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Hydroxychloroquine Sulphate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ibandronate Sodium bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ibrutinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Icatibant Acetate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Imatinib Mesylate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ketorolac Trometamol bulk offered by Dr. Reddy\'s Laboratories
Find a price of Linezolid bulk offered by Dr. Reddy\'s Laboratories
Find a price of Mavacamten bulk offered by Dr. Reddy\'s Laboratories
Find a price of Milnacipran Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Mirogabalin Besylate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Moxifloxacin Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Naproxen bulk offered by Dr. Reddy\'s Laboratories
Find a price of Naproxen Sodium bulk offered by Dr. Reddy\'s Laboratories
Find a price of Naratriptan bulk offered by Dr. Reddy\'s Laboratories
Find a price of Nateglinide bulk offered by Dr. Reddy\'s Laboratories
Find a price of Nicotinamide Riboside Chloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Niraparib Tosylate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Nizatidine bulk offered by Dr. Reddy\'s Laboratories
Find a price of Omeprazole bulk offered by Dr. Reddy\'s Laboratories
Find a price of Paliperidone bulk offered by Dr. Reddy\'s Laboratories
Find a price of Paliperidone Palmitate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Perfluorohexyloctane bulk offered by Dr. Reddy\'s Laboratories
Find a price of Pirtobrutinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Pregabalin bulk offered by Dr. Reddy\'s Laboratories
Find a price of Primidone bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ranitidine Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Resmetirom bulk offered by Dr. Reddy\'s Laboratories
Find a price of Risperidone bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ritlecitinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ritonavir bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ropinirole bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ropinirole Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Rosiglitazone Maleate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Sacubitril-Valsartan bulk offered by Dr. Reddy\'s Laboratories
Find a price of Salcaprozate Sodium bulk offered by Dr. Reddy\'s Laboratories
Find a price of Saxagliptin Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Siponimod bulk offered by Dr. Reddy\'s Laboratories
Find a price of Siponimod fumarate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Sitagliptin Malate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Sugammadex Sodium bulk offered by Dr. Reddy\'s Laboratories
Find a price of Terbinafine Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Ticagrelor bulk offered by Dr. Reddy\'s Laboratories
Find a price of Topiramate bulk offered by Dr. Reddy\'s Laboratories
Find a price of Treprostinil Diolamine bulk offered by Dr. Reddy\'s Laboratories
Find a price of Tucatinib bulk offered by Dr. Reddy\'s Laboratories
Find a price of Udenafil bulk offered by Dr. Reddy\'s Laboratories
Find a price of Valganciclovir Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Valsartan bulk offered by Dr. Reddy\'s Laboratories
Find a price of Venlafaxine Hydrochloride bulk offered by Dr. Reddy\'s Laboratories
Find a price of Voclosporin bulk offered by Dr. Reddy\'s Laboratories
Find a price of Zolmitriptan bulk offered by Dr. Reddy\'s Laboratories
Find a price of Empagliflozin (micronized) bulk offered by Dr. Reddy\'s Laboratories
Find a price of Facebook Start bulk offered by Dr. Reddy\'s Laboratories