14 Apr 2025
// PRESS RELEASE
04 Apr 2025
// EXPRESSPHARMA
22 Feb 2025
// INDPHARMAPOST
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
About
Products listed herein may not be available for commercial use in countries where any relevant third-party intellectual property is in force.
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
Industry Trade Show
Attending
24-26 June, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #9.1A48
28-30 October, 2025
CONTACT DETAILS





Events
Webinars & Exhibitions
CPhI North America CPhI North America
Industry Trade Show
Not Confirmed
20-22 May, 2025
Industry Trade Show
Attending
24-26 June, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #9.1A48
28-30 October, 2025
CORPORATE CONTENT #SupplierSpotlight
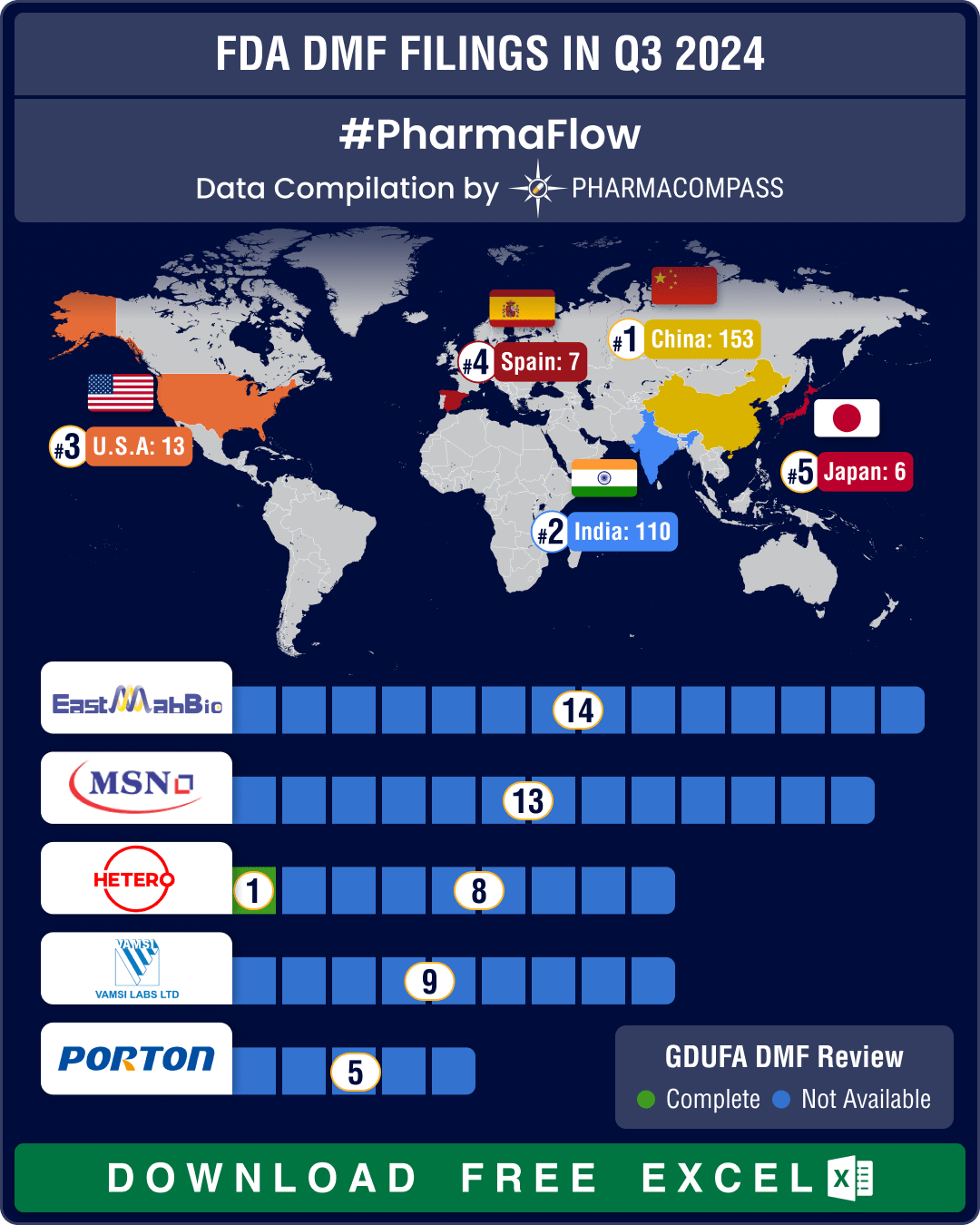
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions

14 Apr 2025
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2025/04/Granules-India-Announces-Closing-of-Acquisition-of-Senn-Chemicals-Strengthening-Capabilities-in-Peptide-Therapeutics-and-CDMO-Services.pdf

04 Apr 2025
// EXPRESSPHARMA
https://www.expresspharma.in/granules-india-and-aig-hospitals-conduct-breast-health-camp-for-women-police-officers-in-telangana/

22 Feb 2025
// INDPHARMAPOST
https://www.indianpharmapost.com/news/granules-india-acquires-swiss-senn-chemicals-to-foray-into-peptide-and-cdmo-business-16813

30 Jan 2025
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2025/01/Granules-Strengthens-ADHD-Portfolio-with-FDA-Approval-for-Lisdexamfetamine-Dimesylate-Capsules.pdf

27 Jan 2025
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2025/01/Press-Release-Q3-FY25.pdf

29 Dec 2024
// BUSINESS STD
https://www.business-standard.com/companies/news/granules-india-expects-fda-nod-for-drug-from-gagillapur-facility-by-fy26-124122900434_1.html
Inspections and registrations
ABOUT THIS PAGE
Granules India Limited is a supplier offers 59 products (APIs, Excipients or Intermediates).
Find a price of Metformin bulk with DMF, CEP, JDMF, WC offered by Granules India Limited
Find a price of Paracetamol bulk with DMF, CEP, JDMF, WC offered by Granules India Limited
Find a price of Atazanavir Sulfate bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Cetirizine Dihydrochloride bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Guaifenesin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Losartan Potassium bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Metformin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Pirfenidone bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Rifaximin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Trazodone Hydrochloride bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Dimethyl Fumarate bulk with DMF, WC offered by Granules India Limited
Find a price of Eltrombopag bulk with DMF, WC offered by Granules India Limited
Find a price of Esomeprazole Magnesium bulk with DMF, CEP offered by Granules India Limited
Find a price of Fexofenadine Hydrochloride bulk with DMF, WC offered by Granules India Limited
Find a price of Levetiracetam bulk with DMF, CEP offered by Granules India Limited
Find a price of Levocetirizine Dihydrochloride bulk with DMF, WC offered by Granules India Limited
Find a price of Metformin bulk with CEP, WC offered by Granules India Limited
Find a price of Methocarbamol bulk with DMF, WC offered by Granules India Limited
Find a price of Metoprolol Succinate bulk with DMF, WC offered by Granules India Limited
Find a price of Omeprazole bulk with DMF, CEP offered by Granules India Limited
Find a price of Omeprazole Magnesium bulk with DMF, WC offered by Granules India Limited
Find a price of Pantoprazole Sodium bulk with DMF, CEP offered by Granules India Limited
Find a price of Rifaximin bulk with DMF, CEP offered by Granules India Limited
Find a price of Sorafenib bulk with DMF, CEP offered by Granules India Limited
Find a price of Bupropion Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Dapagliflozin bulk with DMF offered by Granules India Limited
Find a price of Dasatinib bulk with DMF offered by Granules India Limited
Find a price of Empagliflozin bulk with DMF offered by Granules India Limited
Find a price of Esomeprazole Magnesium bulk with DMF offered by Granules India Limited
Find a price of Gabapentin bulk with WC offered by Granules India Limited
Find a price of Ibuprofen bulk with DMF offered by Granules India Limited
Find a price of Lapatinib Ditosylate bulk with DMF offered by Granules India Limited
Find a price of Linagliptin bulk with DMF offered by Granules India Limited
Find a price of Losartan Potassium bulk with DMF offered by Granules India Limited
Find a price of Metoprolol Succinate bulk with DMF offered by Granules India Limited
Find a price of Nilotinib bulk with DMF offered by Granules India Limited
Find a price of Paracetamol bulk with DMF offered by Granules India Limited
Find a price of Pazopanib Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Prazosin Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Tenofovir Disoproxil Succinate bulk with WC offered by Granules India Limited
Find a price of Vigabatrin bulk with DMF offered by Granules India Limited
Find a price of Avatrombopag Maleate bulk offered by Granules India Limited
Find a price of Bortezomib bulk offered by Granules India Limited
Find a price of Brinzolamide bulk offered by Granules India Limited
Find a price of Cabozantinib bulk offered by Granules India Limited
Find a price of Diphenhydramine Hydrochloride bulk offered by Granules India Limited
Find a price of Elacestrant bulk offered by Granules India Limited
Find a price of Eltrombopag bulk offered by Granules India Limited
Find a price of Guaifenesin bulk offered by Granules India Limited
Find a price of Ibuprofen bulk offered by Granules India Limited
Find a price of Levocetirizine Dihydrochloride bulk offered by Granules India Limited
Find a price of Lisdexamfetamine Dimesylate bulk offered by Granules India Limited
Find a price of Losartan Potassium bulk offered by Granules India Limited
Find a price of Melphalan Hydrochloride bulk offered by Granules India Limited
Find a price of Metformin bulk offered by Granules India Limited
Find a price of Methocarbamol bulk offered by Granules India Limited
Find a price of Paracetamol bulk offered by Granules India Limited
Find a price of Penicillamine bulk offered by Granules India Limited
Find a price of Potassium Chloride bulk offered by Granules India Limited