29 Dec 2024
// BUSINESS STD
17 Dec 2024
// PRESS RELEASE
16 Dec 2024
// FDA
Latest Content by PharmaCompass
 KEY PRODUCTS
KEY PRODUCTS
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
About
Industry Trade Show
Not Confirmed
17-20 March, 2025
Pharma, Lab & Chemical...Pharma, Lab & Chemical Expo
Industry Trade Show
Not Confirmed
03-05 January, 2025
Industry Trade Show
Not Confirmed
07-09 January, 2025
CONTACT DETAILS





Events
Webinars & Exhibitions
Industry Trade Show
Not Confirmed
17-20 March, 2025
Pharma, Lab & Chemical...Pharma, Lab & Chemical Expo
Industry Trade Show
Not Confirmed
03-05 January, 2025
Industry Trade Show
Not Confirmed
07-09 January, 2025
CORPORATE CONTENT #SupplierSpotlight
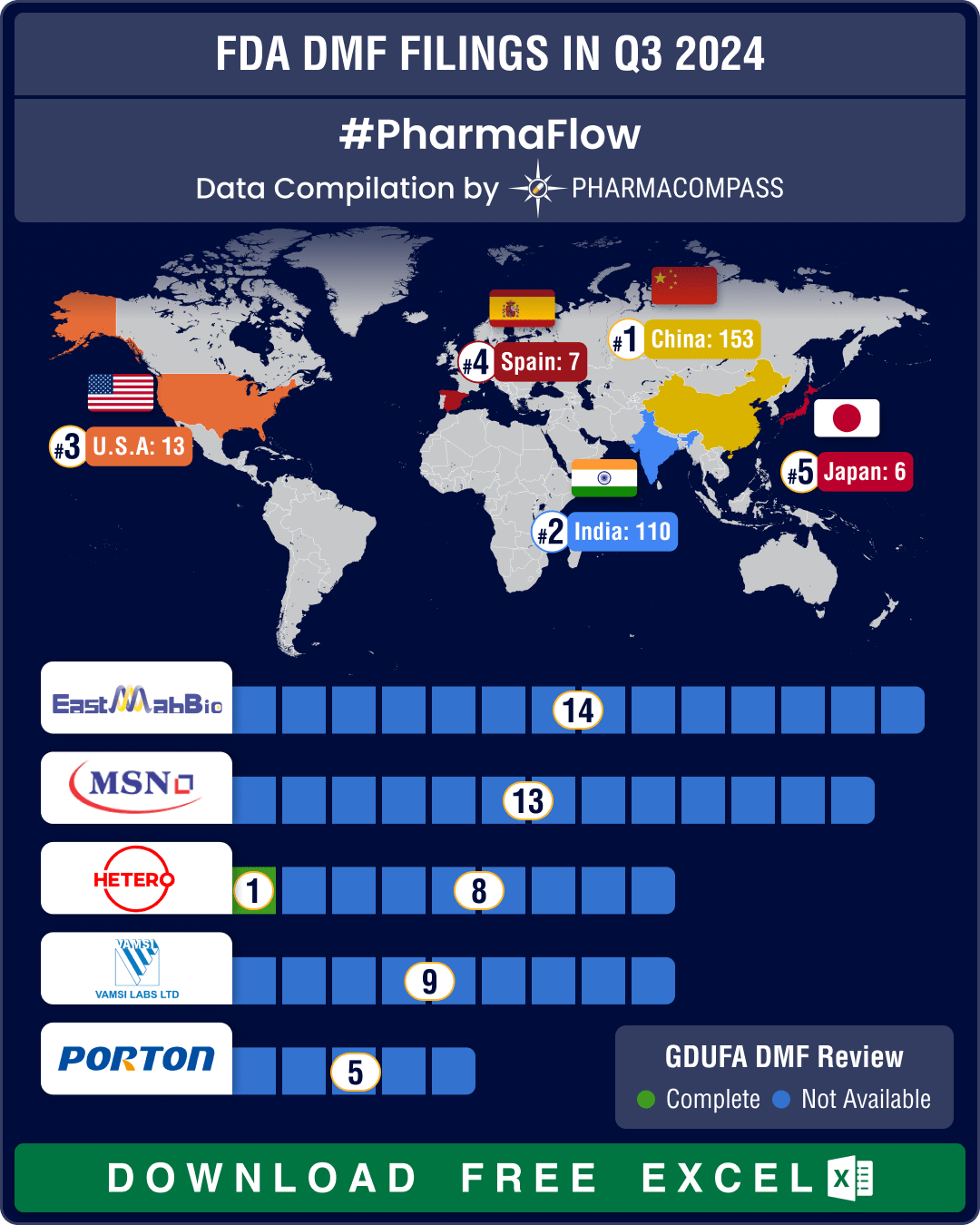
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions

29 Dec 2024
// BUSINESS STD
https://www.business-standard.com/companies/news/granules-india-expects-fda-nod-for-drug-from-gagillapur-facility-by-fy26-124122900434_1.html

17 Dec 2024
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2024/12/Granules-India-Limited-Announces-FDA-Approval-for-ADHD-Treatment-Addressing-Drug-Shortages-in-the-U.S.pdf

16 Dec 2024
// FDA
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=219258

04 Dec 2024
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2024/12/GIL-Confirms-USFDA-Classification-Does-Not-Impact-Ongoing-Operations-Assures-Commitment-to-Compliance-and-Growth.pdf

30 Nov 2024
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2024/11/Granules-Net-Zero-Commitment-Validated-and-Approved-by-Science-Based-Targets-Initiative.pdf

18 Nov 2024
// EXPRESSPHARMA
https://www.expresspharma.in/granules-india-appoints-ovais-sarmad-as-sustainability-advisor/
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2016-07-28
Pay. Date : 2016-07-01
DMF Number : 16625
Submission : 2003-06-06
Status : Active
Type : II

Certificate Number : CEP 2018-120 - Rev 01
Issue Date : 2023-12-12
Type : Chemical
Substance Number : 931
Status : Valid

Registration Number : 305MF10066
Registrant's Address : 2nd Floor, 3rd Block My Home Hub, Madhapur, Hyderabad, Telangana 500081 India
Initial Date of Registration : 2023-05-24
Latest Date of Registration :

Date of Issue : 2022-06-30
Valid Till : 2025-07-02
Written Confirmation Number : WC-0028
Address of the Firm :

NDC Package Code : 62207-012
Start Marketing Date : 2017-10-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Agerson Bio Co., Ltd.
Registration Date : 2023-01-13
Registration Number : 20050831-37-C-34-05(13)
Manufacturer Name : M/s Granules India Limited
Manufacturer Address : Plot No.: 15A/1, Phase-Ⅲ, IDA, Jeedimetla, Quthbullapur (M), Medchal-Malkajgiri District - 500 055 Telangana State, India

| Available Reg. Filing : ROW, ASMF, CA, BR, MX |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17722
Submission : 2004-09-22
Status : Active
Type : II

Certificate Number : R1-CEP 1998-047 - Rev 06
Issue Date : 2018-01-19
Type : Chemical
Substance Number : 49
Status : Valid

Registration Number : 228MF10061
Registrant's Address : 2nd Floor, 3rd Block, My Home Hub, Madhapur, Hyderabad, Telangana 500081 India
Initial Date of Registration : 2016-02-24
Latest Date of Registration :

Date of Issue : 2022-06-29
Valid Till : 2025-07-02
Written Confirmation Number : WC-0116
Address of the Firm :

NDC Package Code : 62207-100
Start Marketing Date : 2009-10-22
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (90mg/100mg)
Marketing Category : DRUG FOR FURTHER PRO...

Registrant Name : Insung Trading Co., Ltd.
Registration Date : 2023-03-29
Registration Number : 20050915-32-C-198-10(B)
Manufacturer Name : Granules India Ltd
Manufacturer Address : H.No.6-5 & 6-11, Temple Road, Bonthapally Village, Gummadidala Mandal, Sangareddy Dist-502313, Telangana State, India.

| Available Reg. Filing : ROW, ASMF, CA, MX |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32259
Submission : 2017-12-27
Status : Active
Type : II

Certificate Number : CEP 2020-279 - Rev 01
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 2898
Status : Valid

Date of Issue : 2022-07-08
Valid Till : 2025-06-28
Written Confirmation Number : WC-0024
Address of the Firm :

NDC Package Code : 62207-005
Start Marketing Date : 2018-06-11
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : EXPORT ONLY

| Available Reg. Filing : ASMF, MX |

GDUFA
DMF Review : Reviewed
Rev. Date : 2016-03-23
Pay. Date : 2015-12-14
DMF Number : 30063
Submission : 2016-02-01
Status : Active
Type : II

Certificate Number : CEP 2009-349 - Rev 02
Issue Date : 2024-10-16
Type : Chemical
Substance Number : 1084
Status : Valid

Date of Issue : 2022-07-08
Valid Till : 2025-06-28
Written Confirmation Number : WC-0024
Address of the Firm :

NDC Package Code : 62207-001
Start Marketing Date : 2016-12-06
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (25kg/1)
Marketing Category : EXPORT ONLY

| Available Reg. Filing : ASMF, CN, ROW |

GDUFA
DMF Review : Reviewed
Rev. Date : 2014-03-19
Pay. Date : 2014-03-11
DMF Number : 14372
Submission : 1999-08-30
Status : Active
Type : II

Certificate Number : R0-CEP 2021-332 - Rev 00
Issue Date : 2021-12-10
Type : Chemical
Substance Number : 615
Status : Valid

Date of Issue : 2022-06-30
Valid Till : 2025-07-02
Written Confirmation Number : WC-0028
Address of the Firm :

NDC Package Code : 62207-500
Start Marketing Date : 2017-08-17
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (95mg/100mg)
Marketing Category : DRUG FOR FURTHER PRO...

Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2021-11-30
Registration Number : 20210309-211-J-857(3)
Manufacturer Name : Granules India Limited
Manufacturer Address : 15A/1, IDA, Phase-III, Jeedimetla, Quthbullapur Mandal, Malkajgiri Dist – 500055, Telangana State, India

| Available Reg. Filing : ASMF, ROW, BR |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20998
Submission : 2007-10-31
Status : Active
Type : II

Certificate Number : R1-CEP 2009-201 - Rev 03
Issue Date : 2018-11-27
Type : Chemical
Substance Number : 615
Status : Valid

Date of Issue : 2022-06-29
Valid Till : 2025-07-02
Written Confirmation Number : WC-0116
Address of the Firm :

NDC Package Code : 62207-500
Start Marketing Date : 2017-08-17
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (95mg/100mg)
Marketing Category : DRUG FOR FURTHER PRO...

Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2021-11-30
Registration Number : 20210309-211-J-857(3)
Manufacturer Name : Granules India Limited
Manufacturer Address : 15A/1, IDA, Phase-III, Jeedimetla, Quthbullapur Mandal, Malkajgiri Dist – 500055, Telangana State, India

| Available Reg. Filing : ASMF, ROW, BR |

GDUFA
DMF Review : Reviewed
Rev. Date : 2020-10-09
Pay. Date : 2020-09-08
DMF Number : 22711
Submission : 2009-04-02
Status : Active
Type : II

Certificate Number : CEP 2020-333 - Rev 03
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 2232
Status : Valid

Date of Issue : 2022-07-08
Valid Till : 2025-06-28
Written Confirmation Number : WC-0024
Address of the Firm :

NDC Package Code : 62207-007
Start Marketing Date : 2018-04-09
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Hiple Co., Ltd.
Registration Date : 2016-05-02
Registration Number : 20100616-122-G-58-21(2)
Manufacturer Name : Granules India Limited (Unit-IV)
Manufacturer Address : Plat No.8, JN. Pharma City, Tadi (V), Parawada Mandal, Visakhapatnam District, Andhra Pradesh India

| Available Reg. Filing : ASMF, CN |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17723
Submission : 2004-09-22
Status : Active
Type : II

Certificate Number : R1-CEP 2004-124 - Rev 07
Issue Date : 2023-05-05
Type : Chemical
Substance Number : 931
Status : Valid

Date of Issue : 2022-06-29
Valid Till : 2025-07-02
Written Confirmation Number : WC-0116
Address of the Firm :

NDC Package Code : 62207-012
Start Marketing Date : 2017-10-24
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Agerson Bio Co., Ltd.
Registration Date : 2023-01-13
Registration Number : 20050831-37-C-34-05(13)
Manufacturer Name : M/s Granules India Limited
Manufacturer Address : Plot No.: 15A/1, Phase-Ⅲ, IDA, Jeedimetla, Quthbullapur (M), Medchal-Malkajgiri District - 500 055 Telangana State, India

| Available Reg. Filing : ROW, ASMF, CA, BR, MX |

GDUFA
DMF Review : Reviewed
Rev. Date : 2021-05-27
Pay. Date : 2021-04-19
DMF Number : 33544
Submission : 2019-03-27
Status : Active
Type : II

Certificate Number : CEP 2019-232 - Rev 02
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 1448
Status : Valid

Date of Issue : 2022-05-02
Valid Till : 2025-05-01
Written Confirmation Number : WC-0526
Address of the Firm :

NDC Package Code : 62207-970
Start Marketing Date : 2021-03-25
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg. Filing : ROW, ASMF |

GDUFA
DMF Review : Reviewed
Rev. Date : 2018-09-19
Pay. Date : 2018-08-03
DMF Number : 30922
Submission : 2016-09-30
Status : Active
Type : II

Certificate Number : CEP 2016-288 - Rev 01
Issue Date : 2024-06-06
Type : Chemical
Substance Number : 2856
Status : Valid

Date of Issue : 2022-07-08
Valid Till : 2025-06-28
Written Confirmation Number : WC-0024
Address of the Firm :

NDC Package Code : 62207-004
Start Marketing Date : 2018-02-20
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : BULK INGREDIENT

| Available Reg. Filing : ROW |
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2013-06-08
Pay. Date : 2012-11-29
DMF Number : 19804
Submission : 2006-09-28
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32259
Submission : 2017-12-27
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2021-05-27
Pay. Date : 2021-04-15
DMF Number : 35694
Submission : 2021-03-29
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2016-03-23
Pay. Date : 2015-12-14
DMF Number : 30063
Submission : 2016-02-01
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20998
Submission : 2007-10-31
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36088
Submission : 2021-07-12
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17723
Submission : 2004-09-22
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19972
Submission : 2006-11-16
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17722
Submission : 2004-09-22
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18978
Submission : 2005-11-28
Status : Active
Type : II
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Certificate Numbers : CEP 2020-279 - Rev 01
Status : Valid
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 2898
Certificate Numbers : CEP 2019-091 - Rev 01
Status : Valid
Issue Date : 2024-06-06
Type : Chemical
Substance Number : 2762
Certificate Numbers : CEP 2009-349 - Rev 02
Status : Valid
Issue Date : 2024-10-16
Type : Chemical
Substance Number : 1084
Certificate Numbers : CEP 2017-240 - Rev 02
Status : Valid
Issue Date : 2024-10-14
Type : Chemical
Substance Number : 1524
Certificate Numbers : R1-CEP 2009-201 - Rev 03
Status : Valid
Issue Date : 2018-11-27
Type : Chemical
Substance Number : 615
Certificate Numbers : R0-CEP 2021-332 - Rev 00
Status : Valid
Issue Date : 2021-12-10
Type : Chemical
Substance Number : 615
Certificate Numbers : CEP 2023-138 - Rev 00
Status : Valid
Issue Date : 2024-07-05
Type : Chemical
Substance Number : 2535
Certificate Numbers : CEP 2020-333 - Rev 03
Status : Valid
Issue Date : 2024-06-10
Type : Chemical
Substance Number : 2232
Certificate Numbers : R1-CEP 2004-124 - Rev 07
Status : Valid
Issue Date : 2023-05-05
Type : Chemical
Substance Number : 931
Certificate Numbers : CEP 2018-120 - Rev 01
Status : Valid
Issue Date : 2023-12-12
Type : Chemical
Substance Number : 931
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Insung Trading Co., Ltd.
Registration Date : 2023-03-29
Registration Number : 20050915-32-C-198-10(B)
Manufacturer Name : Granules India Ltd
Manufacturer Address : H.No.6-5 & 6-11, Temple Road, Bonthapally Village, Gummadidala Mandal, Sangareddy Dis...
Registrant Name : Korea Johnson & Johnson Sales Co., Ltd.
Registration Date : 2023-03-03
Registration Number : 20050915-32-C-198-10(A)
Manufacturer Name : Granules India Ltd
Manufacturer Address : H.No.6-5 & 6-11, Temple Road, Bonthapally Village, Gummadidala Mandal, Sangareddy Dis...
Registrant Name : Daeshin Pharmaceutical Co., Ltd.
Registration Date : 2005-09-15
Registration Number : 20050915-32-C-198-10
Manufacturer Name : Granules India Ltd
Manufacturer Address : H. No.: 6-5 & 6-11, Temple Road, Bonthapally Village, Gummadidala Mandal, Sangareddy ...
Registrant Name : Insung Trading Co., Ltd.
Registration Date : 2021-03-09
Registration Number : 20210309-211-J-857
Manufacturer Name : Granules India Limited
Manufacturer Address : 15A/1, IDA, Phase-III, Jeedimetla, Quthbullapur Mandal, Malkajgiri Dist – 500055, T...
Registrant Name : Daeshin Pharmaceutical Co., Ltd.
Registration Date : 2021-07-05
Registration Number : 20210309-211-J-857(2)
Manufacturer Name : Granules India Limited
Manufacturer Address : 15A/1, IDA, Phase-III, Jeedimetla, Quthbullapur Mandal, Malkajgiri Dist – 500055, T...
Registrant Name : IMCD Korea Co., Ltd.
Registration Date : 2021-06-04
Registration Number : 20210309-211-J-857(1)
Manufacturer Name : Granules India Limited
Manufacturer Address : 15A/1, IDA, Phase-III, Jeedimetla, Quthbullapur Mandal, Malkajgiri Dist-500 055.,Tela...
Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2021-11-30
Registration Number : 20210309-211-J-857(3)
Manufacturer Name : Granules India Limited
Manufacturer Address : 15A/1, IDA, Phase-III, Jeedimetla, Quthbullapur Mandal, Malkajgiri Dist – 500055, T...
Registrant Name : Dongkwang Pharmaceutical Co., Ltd.
Registration Date : 2016-02-25
Registration Number : 20090720-40-C-258-15(2)
Manufacturer Name : Granules India Limited
Manufacturer Address : Plot No 8, Jawaharlal Nehru Pharma City, Tadi Village, Parawada Mandal, Vitsakhapatna...
Registrant Name : Kukjeon Pharmaceutical Co., Ltd.
Registration Date : 2009-07-20
Registration Number : 20090720-40-C-258-15
Manufacturer Name : Granules India Limited_x000D_
Manufacturer Address : Plot No 8, Jawaharlal Nehru Pharma City, Tadi Village, Parawada Mandal, Vitsakhapatna...
Registrant Name : Hiple Co., Ltd.
Registration Date : 2016-05-02
Registration Number : 20090720-40-C-258-15(3)
Manufacturer Name : Granules India Limited
Manufacturer Address : Plot No 8, Jawaharlal Nehru Pharma City, Tadi Village, Parawada Mandal, Vitsakhapatna...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Details:
Cuvposa is an anticholinergic indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling.
Lead Product(s): Glycopyrronium Bromide
Therapeutic Area: Neurology Brand Name: Cuvposa-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 20, 2024
Lead Product(s) : Glycopyrronium Bromide
Therapeutic Area : Neurology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Gets USFDA Nod for Generic Glycopyrrolate Oral Solution
Details : Cuvposa is an anticholinergic indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling.
Brand Name : Cuvposa-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 20, 2024
Details:
Desyrel-Generic (trazodone hydrochloride) is a selective serotonin reuptake inhibitor indicated for the treatment of patients with major depressive disorder.
Lead Product(s): Trazodone Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Desyrel-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 09, 2024
Lead Product(s) : Trazodone Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Gains ANDA approval for Trazodone Tablets
Details : Desyrel-Generic (trazodone hydrochloride) is a selective serotonin reuptake inhibitor indicated for the treatment of patients with major depressive disorder.
Brand Name : Desyrel-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 09, 2024
Details:
Pantoprazole Sodium Delayed-Release tablets are indicated for short-term treatment of Erosive Esophagitis Associated with GERD and Pathological Hypersecretory Conditions.
Lead Product(s): Pantoprazole Sodium
Therapeutic Area: Gastroenterology Brand Name: Protonix-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 13, 2023
Lead Product(s) : Pantoprazole Sodium
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Limited Received ANDA Approval for Pantoprazole Sod Delayed-Release Tablets
Details : Pantoprazole Sodium Delayed-Release tablets are indicated for short-term treatment of Erosive Esophagitis Associated with GERD and Pathological Hypersecretory Conditions.
Brand Name : Protonix-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 13, 2023
Details:
Sildenafil is an inhibitor of cyclic GMP specific PDE type 5, the predominant enzyme metabolizing cyclic GMP in the corpus cavernosum. It is indicated for pulmonary arterial hypertension in adults.
Lead Product(s): Sildenafil Citrate
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Revatio-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 03, 2023
Lead Product(s) : Sildenafil Citrate
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Limited Received ANDA Approval for Sildenafil for Oral Suspension
Details : Sildenafil is an inhibitor of cyclic GMP specific PDE type 5, the predominant enzyme metabolizing cyclic GMP in the corpus cavernosum. It is indicated for pulmonary arterial hypertension in adults.
Brand Name : Revatio-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
December 03, 2023
Details:
Esomeprazole magnesium delayed-release capsules is a proton pump inhibitor indicated for the treatment of gastroesophageal reflux disease and NSAID-associated gastric ulcer.
Lead Product(s): Esomeprazole Magnesium
Therapeutic Area: Gastroenterology Brand Name: Nexium-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 19, 2023
Lead Product(s) : Esomeprazole Magnesium
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Gets USFDA Nod for Generic Drug Used for Short-term Treatment of Heartburn
Details : Esomeprazole magnesium delayed-release capsules is a proton pump inhibitor indicated for the treatment of gastroesophageal reflux disease and NSAID-associated gastric ulcer.
Brand Name : Nexium-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 19, 2023
Details:
Generic of Losartan potassium and hydrochlorothiazide tablets are been approved by FDA, indicated for hypertension to lower blood pressure and to reduce the risk of stroke.
Lead Product(s): Losartan Potassium,Hydrochlorothiazide
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Hyzaar-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 30, 2023
Lead Product(s) : Losartan Potassium,Hydrochlorothiazide
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Limited Received ANDA Approval for Losartan and Hydrochlorothiazide Tablets
Details : Generic of Losartan potassium and hydrochlorothiazide tablets are been approved by FDA, indicated for hypertension to lower blood pressure and to reduce the risk of stroke.
Brand Name : Hyzaar-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 30, 2023
Details:
The company has received approval from the US Food and Drug Administration (USFDA) for generic version for Acetaminophen and Ibuprofen tablets (250 mg/125 mg) indicated for pain and inflammation.
Lead Product(s): Ibuprofen
Therapeutic Area: Neurology Brand Name: Advil-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable July 14, 2023
FDA Approves Granules' OTC Equivalent Of Advil Dual Action Tablets
Details : The company has received approval from the US Food and Drug Administration (USFDA) for generic version for Acetaminophen and Ibuprofen tablets (250 mg/125 mg) indicated for pain and inflammation.
Brand Name : Advil-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
July 14, 2023
Details:
FDA approved generic of Levetiracetam for seizures. It prevents seizure activity via selective inhibition of hypersynchronized epileptiform burst firing without affecting normal neuronal transmission.
Lead Product(s): Levetiracetam
Therapeutic Area: Neurology Brand Name: Keppra-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 14, 2023
Lead Product(s) : Levetiracetam
Therapeutic Area : Neurology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Limited Receives ANDA Approval for Levetiracetam Tablets in record 9 months
Details : FDA approved generic of Levetiracetam for seizures. It prevents seizure activity via selective inhibition of hypersynchronized epileptiform burst firing without affecting normal neuronal transmission.
Brand Name : Keppra-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 14, 2023
Details:
Generic version of Metoprolol Succinate ER Tablets have been approved, these are catecholamines inhibitor indicated for the treatment of hypertension in order to lower blood pressure.
Lead Product(s): Metoprolol Succinate
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Toprol-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 13, 2023
Lead Product(s) : Metoprolol Succinate
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Limited Received ANDA Approval for Metoprolol Succinate ER Tablets
Details : Generic version of Metoprolol Succinate ER Tablets have been approved, these are catecholamines inhibitor indicated for the treatment of hypertension in order to lower blood pressure.
Brand Name : Toprol-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 13, 2023
Details:
Generic version of Venlafaxine HCl extended-release capsules has been approved which are indicated for the treatment of MDD, GAD, SAD and Panic Disorder (PD).
Lead Product(s): Venlafaxine Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Effexor-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 19, 2023
Lead Product(s) : Venlafaxine Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Limited Received ANDA Approval for Venlafaxine ER Capsules
Details : Generic version of Venlafaxine HCl extended-release capsules has been approved which are indicated for the treatment of MDD, GAD, SAD and Panic Disorder (PD).
Brand Name : Effexor-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 19, 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]RLD : No
TE Code :
Dosage Form : TABLET, EXTENDED RELEASE; ORAL
Proprietary Name : ACETAMINOPHEN
Dosage Strength : 650MG
Approval Date : 2019-04-16
Application Number : 211544
RX/OTC/DISCN : OTC
RLD : No
TE Code :
RLD : No
TE Code :
ACETAMINOPHEN; ASPIRIN; CAFFEINE
Dosage Form : TABLET; ORAL
Proprietary Name : ACETAMINOPHEN, ASPIRIN A...
Dosage Strength : 250MG;250MG;65MG
Approval Date : 2021-02-23
Application Number : 214039
RX/OTC/DISCN : OTC
RLD : No
TE Code :
RLD : No
TE Code : AA
Dosage Form : CAPSULE; ORAL
Proprietary Name : BUTALBITAL AND ACETAMINO...
Dosage Strength : 300MG;50MG
Approval Date : 2019-11-22
Application Number : 213115
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
RLD : No
TE Code : AA
ACETAMINOPHEN; BUTALBITAL; CAFFEINE
Dosage Form : TABLET; ORAL
Proprietary Name : BUTALBITAL, ACETAMINOPHE...
Dosage Strength : 325MG;50MG;40MG
Approval Date : 2008-12-01
Application Number : 40864
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
RLD : No
TE Code : AA
ACETAMINOPHEN; BUTALBITAL; CAFFEINE
Dosage Form : CAPSULE; ORAL
Proprietary Name : BUTALBITAL, ACETAMINOPHE...
Dosage Strength : 300MG;50MG;40MG
Approval Date : 2020-04-08
Application Number : 213321
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
RLD : No
TE Code : AA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Dosage Form : TABLET; ORAL
Proprietary Name : HYDROCODONE BITARTRATE A...
Dosage Strength : 325MG;5MG
Approval Date : 2020-01-03
Application Number : 211729
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
RLD : No
TE Code : AA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Dosage Form : TABLET; ORAL
Proprietary Name : HYDROCODONE BITARTRATE A...
Dosage Strength : 325MG;7.5MG
Approval Date : 2020-01-03
Application Number : 211729
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
RLD : No
TE Code : AA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Dosage Form : TABLET; ORAL
Proprietary Name : HYDROCODONE BITARTRATE A...
Dosage Strength : 325MG;10MG
Approval Date : 2020-01-03
Application Number : 211729
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
RLD : No
TE Code :
Dosage Form : TABLET; ORAL
Proprietary Name : ACETAMINOPHEN AND IBUPRO...
Dosage Strength : 250MG;125MG
Approval Date : 2023-07-13
Application Number : 216592
RX/OTC/DISCN : OTC
RLD : No
TE Code :
RLD : No
TE Code : AA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Dosage Form : TABLET; ORAL
Proprietary Name : OXYCODONE AND ACETAMINOP...
Dosage Strength : 325MG;2.5MG
Approval Date : 2019-10-31
Application Number : 211708
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
Inspections and registrations
District Decision : No Action Indicated
Inspection End Date : 2009-11-12
City : Qutbullapur Mandal, Ranga...
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2009-11-12
District Decision : No Action Indicated
Inspection End Date : 2010-11-22
City : Bonthapally, Medak Distri...
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2010-11-22
District Decision : Voluntary Action Indicated
Inspection End Date : 2012-09-13
City : Qutbullapur Mandal, Ranga...
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2012-09-13
District Decision : No Action Indicated
Inspection End Date : 2013-06-14
City : Visakhapatnam
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2013-06-14
District Decision : No Action Indicated
Inspection End Date : 2014-06-13
City : Bonthapally, Medak Distri...
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2014-06-13
District Decision : No Action Indicated
Inspection End Date : 2015-03-13
City : Qutbullapur Mandal, Ranga...
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2015-03-13
District Decision : Voluntary Action Indicated
Inspection End Date : 2013-05-08
City : Hyderabad
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2013-05-08
District Decision : Voluntary Action Indicated
Inspection End Date : 2009-03-06
City : Hyderabad
State :
Country/Area : IN
Zip :
District : ORA
Center : CDER
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2009-03-06
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Type : GMP Certificates
Number : FI21/01/201...
EudraGMDP Key : 21874
Country : India
Issue Date : 2013-12-16
Post Code : 502313
NCA Ref : 1.04721299e+...
City : Medak District
Type : GMP Certificates
Number : FI22/01/201...
EudraGMDP Key : 21875
Country : India
Issue Date : 2013-12-16
Post Code : 500 043
NCA Ref : 104621298630...
City : Quthbullapur Mandal, Ra...
Type : GMP Certificates
Number : FT052/S1/MH...
EudraGMDP Key : 31856
Country : India
Issue Date : 2015-10-26
Post Code : 500 055
NCA Ref : 104621298632...
City : Hyderabad
Type : GMP Certificates
Number : FI21/01/201...
EudraGMDP Key : 25980
Country : India
Issue Date : 2014-10-27
Post Code : 502313
NCA Ref : 104721298997...
City : Medak District
Type : GMP Certificates
Number : HMP/PT/221/...
EudraGMDP Key : 28131
Country : India
Issue Date : 2014-11-19
Post Code : 500055
NCA Ref : A0372917466
City : HYDERABAD, ANDHRA PRADE...
Type : GMP Certificates
Number : FT022/MH/00...
EudraGMDP Key : 40343
Country : India
Issue Date : 2017-02-24
Post Code : 500043
NCA Ref : FT022
City : Ranga Reddy District
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
Granules India Limited is a supplier offers 62 products (APIs, Excipients or Intermediates).
Find a price of Metformin bulk with DMF, CEP, JDMF, WC offered by Granules India Limited
Find a price of Paracetamol bulk with DMF, CEP, JDMF, WC offered by Granules India Limited
Find a price of Atazanavir Sulfate bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Cetirizine Dihydrochloride bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Guaifenesin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Losartan Potassium bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Metformin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Metoprolol Succinate bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Pirfenidone bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Rifaximin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Trazodone Hydrochloride bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Capecitabine bulk with CEP, WC offered by Granules India Limited
Find a price of Dimethyl Fumarate bulk with DMF, WC offered by Granules India Limited
Find a price of Eltrombopag bulk with DMF, WC offered by Granules India Limited
Find a price of Fexofenadine Hydrochloride bulk with DMF, WC offered by Granules India Limited
Find a price of Gliclazide bulk with CEP, WC offered by Granules India Limited
Find a price of Levetiracetam bulk with DMF, CEP offered by Granules India Limited
Find a price of Levocetirizine Dihydrochloride bulk with DMF, WC offered by Granules India Limited
Find a price of Methocarbamol bulk with DMF, WC offered by Granules India Limited
Find a price of Omeprazole bulk with DMF, CEP offered by Granules India Limited
Find a price of Omeprazole Magnesium bulk with DMF, WC offered by Granules India Limited
Find a price of Pantoprazole Sodium bulk with DMF, CEP offered by Granules India Limited
Find a price of Penicillamine bulk with CEP, WC offered by Granules India Limited
Find a price of Rifaximin bulk with DMF, CEP offered by Granules India Limited
Find a price of Abacavir Sulfate bulk with WC offered by Granules India Limited
Find a price of Azathioprine bulk with WC offered by Granules India Limited
Find a price of Azithromycin bulk with WC offered by Granules India Limited
Find a price of Brinzolamide bulk with WC offered by Granules India Limited
Find a price of Bupropion Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Clopidogrel bulk with WC offered by Granules India Limited
Find a price of Dapagliflozin bulk with DMF offered by Granules India Limited
Find a price of Darunavir bulk with WC offered by Granules India Limited
Find a price of Dasatinib bulk with DMF offered by Granules India Limited
Find a price of Empagliflozin bulk with DMF offered by Granules India Limited
Find a price of Esomeprazole Magnesium bulk with DMF offered by Granules India Limited
Find a price of Fluconazole bulk with WC offered by Granules India Limited
Find a price of Gabapentin bulk with WC offered by Granules India Limited
Find a price of Itraconazole bulk with WC offered by Granules India Limited
Find a price of Lapatinib Ditosylate bulk with DMF offered by Granules India Limited
Find a price of Linagliptin bulk with DMF offered by Granules India Limited
Find a price of Losartan Potassium bulk with DMF offered by Granules India Limited
Find a price of Metformin bulk with WC offered by Granules India Limited
Find a price of Metoprolol Succinate bulk with DMF offered by Granules India Limited
Find a price of Mycophenolate Mofetil bulk with WC offered by Granules India Limited
Find a price of Naproxen Sodium bulk with WC offered by Granules India Limited
Find a price of Nilotinib bulk with DMF offered by Granules India Limited
Find a price of Olmesartan Medoxomil bulk with WC offered by Granules India Limited
Find a price of Paracetamol bulk with DMF offered by Granules India Limited
Find a price of Pazopanib Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Prazosin Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Sorafenib bulk with DMF offered by Granules India Limited
Find a price of Teneligliptin Hydrobromide bulk with WC offered by Granules India Limited
Find a price of Tenofovir Disoproxil Succinate bulk with WC offered by Granules India Limited
Find a price of Tizanidine HCl bulk with WC offered by Granules India Limited
Find a price of Vigabatrin bulk with DMF offered by Granules India Limited
Find a price of Viloxazine Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Avatrombopag Maleate bulk offered by Granules India Limited
Find a price of Cabozantinib bulk offered by Granules India Limited
Find a price of Elacestrant bulk offered by Granules India Limited
Find a price of Ruxolitinib Phosphate bulk offered by Granules India Limited
Find a price of Sitagliptin Phosphate bulk offered by Granules India Limited
Find a price of Tenofovir Alafenamide Fumarate bulk offered by Granules India Limited


LOOKING FOR A SUPPLIER?