25 Nov 2024
// PRESS RELEASE
09 Jan 2025
// PRESS RELEASE
09 Jan 2025
// PRESS RELEASE
25 Nov 2024
// PRESS RELEASE
09 Jan 2025
// PRESS RELEASE
Latest Content by PharmaCompass

 KEY PRODUCTS
KEY PRODUCTS

![]() Reset all filters
Reset all filters
01 1Hyaluronic Acid
02 14Sodium Hyaluronate

![]() Reset all filters
Reset all filters
01 3Active
02 12Blank
01 2Valid
02 13Blank

![]() Reset all filters
Reset all filters
01 1217MF10438
02 1217MF10439
03 1217MF10440
04 1227MF10163
05 1228MF10141
06 1302MF10032
07 9Blank

![]() Reset all filters
Reset all filters
01 15Blank

![]() Reset all filters
Reset all filters
01 120071228-96-E-23-06
02 120071228-96-E-23-06(1)
03 120071228-96-E-23-06(3)
04 120071228-96-E-23-06(4)
05 120071228-96-E-23-06(5)
06 120071228-96-E-23-06(6)
07 120071228-96-E-23-06(7)
08 120071228-96-E-24-07
09 120071228-96-E-24-07(1)
10 120071228-96-E-25-08
11 120071228-96-E-25-08(2)
12 120071228-96-E-25-08(3)
13 120071228-96-E-25-08(5)
14 120191112-96-E-159-47
15 1Blank

![]() Reset all filters
Reset all filters
01 15Blank
01 15Blank

 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 14727
Submission : 2000-02-29
Status : Active
Type : II

Certificate Number : CEP 2018-037 - Rev 02
Issue Date : 2025-02-21
Type : Chemical and TSE
Substance Number : 1472
Status : Valid

Registration Number : 228MF10141
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2016-08-02
Latest Date of Registration :

Registrant Name : Daeshin Pharmaceutical Co., Ltd.
Registration Date : 2023-06-22
Registration Number : 20071228-96-E-25-08(5)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315

| Available Reg. Filing : CN |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 30316
Submission : 2016-04-22
Status : Active
Type : II

Certificate Number : R0-CEP 2019-064 - Rev 00
Issue Date : 2020-04-16
Type : Chemical and TSE
Substance Number : 1472
Status : Valid

Registration Number : 227MF10163
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2015-06-18
Latest Date of Registration :

Registrant Name : Daeshin Pharmaceutical Co., Ltd.
Registration Date : 2019-11-12
Registration Number : 20191112-96-E-159-47
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA Plant
Manufacturer Address : 1800, Aza-Oosaki, Ooaza-Kotesashi, Goka-machi, Sashima-gun Ibaraki-ken, 306-0315, Japan

| Available Reg. Filing : CN |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33346
Submission : 2018-12-31
Status : Active
Type : II

Registration Number : 217MF10440
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2005-08-19
Latest Date of Registration :

Registrant Name : Optus Pharmaceutical Co., Ltd.
Registration Date : 2017-09-11
Registration Number : 20071228-96-E-23-06(7)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315

| Available Reg. Filing : CN |

Registration Number : 302MF10032
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2020-02-27
Latest Date of Registration :

| Available Reg. Filing : CN |

Registration Number : 217MF10438
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2005-08-19
Latest Date of Registration :

Registrant Name : Taejun Pharmaceutical Co., Ltd.
Registration Date : 2012-05-25
Registration Number : 20071228-96-E-23-06(6)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315

| Available Reg. Filing : CN |

Registration Number : 217MF10439
Registrant's Address : Shibuya 1-4-13, Shibuya-ku, Tokyo
Initial Date of Registration : 2005-08-19
Latest Date of Registration :

Registrant Name : Dongkwang Pharmaceutical Co., Ltd.
Registration Date : 2011-07-15
Registration Number : 20071228-96-E-23-06(3)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315

| Available Reg. Filing : CN |

Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2011-07-15
Registration Number : 20071228-96-E-23-06(4)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315

| Available Reg. Filing : CN |

Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2011-07-15
Registration Number : 20071228-96-E-24-07(1)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315

| Available Reg. Filing : CN |

Registrant Name : Dongkwang Pharmaceutical Co., Ltd.
Registration Date : 2011-07-15
Registration Number : 20071228-96-E-25-08(2)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315

| Available Reg. Filing : CN |

Registrant Name : Unimed Pharmaceutical Co., Ltd.
Registration Date : 2011-07-15
Registration Number : 20071228-96-E-25-08(3)
Manufacturer Name : Kewpie Corporation Fine Chemical Division GOKA PLANT
Manufacturer Address : 1800, AZA-OOSAKI, OOAZA-KOTESASHI, GOKA-MACHI, SASHIMA-GUN, IBARAKI-KEN, 306-0315_x000D_

| Available Reg. Filing : CN |
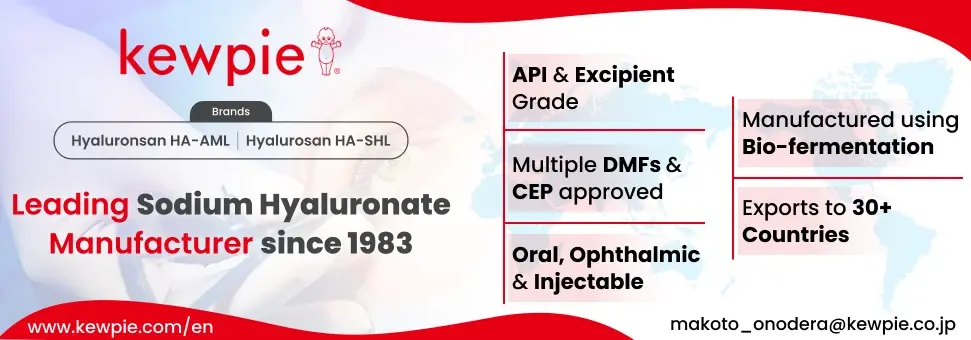
Kewpie Corporation is a supplier offers 5 products (APIs, Excipients or Intermediates).
Find a price of Sodium Hyaluronate bulk with DMF, CEP, JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk with DMF, JDMF offered by Kewpie Corporation
Find a price of Hyaluronic Acid bulk with JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk with JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk offered by Kewpie Corporation