
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Apresoline
2. Apressin
3. Apressoline
4. Hydralazine Hydrochloride
5. Hydralazine Mono Hydrochloride
6. Hydralazine Mono-hydrochloride
7. Hydrallazin
8. Hydrazinophthalazine
9. Hydrochloride, Hydralazine
10. Mono-hydrochloride, Hydralazine
11. Nepresol
1. 1-hydrazinophthalazine
2. 86-54-4
3. Hydralazin
4. 1-hydrazinylphthalazine
5. Hypophthalin
6. Hydrazinophthalazine
7. Apresoline
8. Apresolin
9. Apressin
10. Aprezolin
11. Hidralazin
12. Idralazina
13. Phthalazin-1-ylhydrazine
14. 6-hydralazine
15. Hidralazina
16. Hipoftalin
17. Hydrallazine
18. 1(2h)-phthalazinone, Hydrazone
19. 1-phthalazinylhydrazine
20. 59275-69-3
21. Hydralazinum
22. Hydrazone 1(2h)-phthalazinone
23. (e)-1-hydrazono-1,2-dihydrophthalazine
24. Ciba 5968
25. Apressin (pharmaceutical)
26. Phthalazine, 1-hydrazino-
27. Ba 5968
28. Praparat 5968
29. (2h)-phthalazinone Hydrazone
30. C-5968
31. (1z)-1(2h)-phthalazinone Hydrazone
32. C 5968
33. Hydralazine (inn)
34. Nsc 126699
35. Chebi:5775
36. Nsc-126699
37. 26nak24ls8
38. Apresolin; Apresoline; Apressin
39. C-5068
40. Idralazina [dcit]
41. Idralazina [italian]
42. Hidralazina [spanish]
43. Hydralazine [inn]
44. Hydralazine [inn:ban]
45. Hydralazinum [inn-latin]
46. Hidralazina [inn-spanish]
47. 1(2h)-phthalazinone Hydrazone
48. Hidral
49. Hidral (tn)
50. Ccris 5385
51. Ncgc00015501-02
52. Cas-304-20-1
53. Einecs 201-680-3
54. Brn 0132615
55. Unii-26nak24ls8
56. Phthalazone Hydrazone
57. Hydralazine Polistirex
58. 1-hydrazonophthalazine
59. Spectrum_000875
60. (1e)-1-hydrazono-1,2-dihydrophthalazine
61. Hydralazine [mi]
62. Prestwick0_000169
63. Prestwick1_000169
64. Prestwick2_000169
65. Spectrum2_000969
66. Spectrum3_000455
67. Spectrum4_000005
68. Spectrum5_000822
69. Lopac-h-1753
70. Ec-000.1838
71. Hydralazine [iarc]
72. Epitope Id:137349
73. Hydralazine [vandf]
74. Schembl7810
75. Nciopen2_001484
76. Lopac0_000593
77. Oprea1_207681
78. Oprea1_416878
79. Bspbio_002130
80. Hydralazine [who-dd]
81. Kbiogr_000349
82. Kbioss_001355
83. Wln: T66 Cnnj Bmz
84. 5-25-17-00412 (beilstein Handbook Reference)
85. Divk1c_000117
86. Spbio_000977
87. Spbio_001958
88. Discontinued See: H716531
89. Chembl276832
90. Gtpl7326
91. Dtxsid4023129
92. Bdbm81461
93. Hy-b0464a
94. Kbio1_000117
95. Kbio2_001355
96. Kbio2_003923
97. Kbio2_006491
98. Kbio3_001350
99. Rptusvtufvmdqk-uhfffaoysa-
100. 1(2h)-phthalazinone Hydrazone #
101. Ninds_000117
102. Albb-023848
103. Nsc_3637
104. Nsc126699
105. Stk246900
106. Zinc12360535
107. Hydralazine; Phthalazin-1-ylhydrazine
108. Akos000122609
109. Akos016843064
110. Akos028109138
111. Ccg-204682
112. Db01275
113. Sdccgsbi-0050575.p005
114. Cas_86-54-4
115. Idi1_000117
116. 1-hydrazinylidene-1,2-dihydrophthalazine
117. Ncgc00015501-01
118. Ncgc00015501-03
119. Ncgc00015501-04
120. Ncgc00015501-05
121. Ncgc00015501-06
122. Ncgc00015501-07
123. Ncgc00015501-17
124. Ncgc00162199-01
125. Phthalazine, 1-hydrazino-, Hydrochloride
126. Hlz
127. Ls-13412
128. Sbi-0050575.p004
129. Cs-0013620
130. Ft-0669282
131. (1e)-1-hydrazinylidene-1,2-dihydrophthalazine
132. C07040
133. D08044
134. Ab00053483_03
135. Ab01274815-01
136. Ab01274815_02
137. 599h497
138. A914301
139. A916276
140. Q419987
141. Brd-k82103381-003-03-7
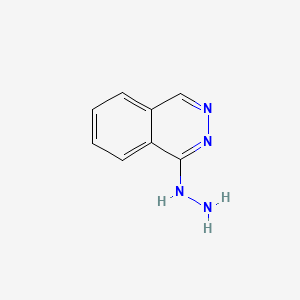
| Molecular Weight | 160.18 g/mol |
|---|---|
| Molecular Formula | C8H8N4 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 160.074896272 g/mol |
| Monoisotopic Mass | 160.074896272 g/mol |
| Topological Polar Surface Area | 63.8 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 150 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Hydralazine is indicated alone or adjunct to standard therapy to treat essential hypertension. A combination product with isosorbide dinitrate is indicated as an adjunct therapy in the treatment of heart failure.
Hydralazine interferes with calcium transport to relax arteriolar smooth muscle and lower blood pressure. Hydralazine has a short duration of action of 2-6h. This drug has a wide therapeutic window, as patients can tolerate doses of up to 300mg. Patients should be cautioned regarding the risk of developing systemic lupus erythematosus syndrome.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C02 - Antihypertensives
C02D - Arteriolar smooth muscle, agents acting on
C02DB - Hydrazinophthalazine derivatives
C02DB02 - Hydralazine
Absorption
Taking oral hydralazine with food improves the bioavailability of the drug. An intravenous dose of 0.3mg/kg leads to an AUC of 17.5-29.4M\*min and a 1mg/kg oral dose leads to an AUC of 4.0-30.4M\*min. The Cmax of oral hydralazine is 0.12-1.31M depending on the acetylator status of patients.
Route of Elimination
<10% of hydralazine is recovered in the feces; 65-90% is recovered in the urine.
Volume of Distribution
The volume of distribution is 1.340.79L/kg in congestive heart failure patients and 1.980.22L/kg in hypertensive patients.
Clearance
The majority of hydralazine clearance is extrahepatic- 55% for rapid acetylators and 70% for slow acetylators. The average clearance in congestive heart failure patients is 1.770.48L/kg/h, while hypertensive patients have an average clearance of 42.78.9mL/min/kg.
Acetylation is a minor metabolic pathway for hydralazine; the major pathway is hydroxylation followed by glucuronidation. There are 5 identified metabolic pathways for hydralazine. Hydralazine can be metabolized to phthalazine or -ketoglutarate hydrazone. These metabolites can be further converted to phthalazinone or hydralazine can be metabolized directly to phthalazinone. Hydralazine can undergo a reversible converstion to the active hydralazine acetone hydrazone. Hydralazine is spontaneously converted to the active pyruvic acid hydrazone or the pyruvic acid hydrazone tricyclic dehydration product, and these metabolites can convert back and forth between these 2 forms. Hydralazine can be converted to hydrazinophthalazinone, which is further converted to the active acetylhydrazinophthalazinone. The final metabolic process hydralazine can undergo is the conversion to an unnamed hydralazine metabolite, which is further metabolized to 3-methyl-s-triazolophthalazine (MTP). MTP can be metabolized to 9-hydroxy-methyltriazolophthalazine or 3-hydroxy-methyltriazolophthalazine; the latter is converted to triazolophthalazine.
Hydralazine has known human metabolites that include hydralazine N-acetyl.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Hydralazine has a half life of 2.2-7.8h in rapid acetylators and 2.0-5.8h in slow acetylators. The half life in heart failure patients is 57-241 minutes with an average of 105 minutes and in hypertensive patients is 200 minutes for rapid acetylators and 297 minutes for slow acetylators.
Hydralazine may interfere with calcium transport in vascular smooth muscle by an unknown mechanism to relax arteriolar smooth muscle and lower blood pressure. The interference with calcium transport may be by preventing influx of calcium into cells, preventing calcium release from intracellular compartments, directly acting on actin and myosin, or a combination of these actions. This decrease in vascular resistance leads to increased heart rate, stroke volume, and cardiac output. Hydralazine also competes with protocollagen prolyl hydroxylase (CPH) for free iron. This competition inhibits CPH mediated hydroxylation of HIF-1, preventing the degradation of HIF-1. Induction of HIF-1 and VEGF promote proliferation of endothelial cells and angiogenesis.
Global Sales Information

Market Place

ABOUT THIS PAGE
14
PharmaCompass offers a list of Hydralazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hydralazine manufacturer or Hydralazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hydralazine manufacturer or Hydralazine supplier.
PharmaCompass also assists you with knowing the Hydralazine API Price utilized in the formulation of products. Hydralazine API Price is not always fixed or binding as the Hydralazine Price is obtained through a variety of data sources. The Hydralazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hydralazine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hydralazine, including repackagers and relabelers. The FDA regulates Hydralazine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hydralazine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hydralazine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hydralazine supplier is an individual or a company that provides Hydralazine active pharmaceutical ingredient (API) or Hydralazine finished formulations upon request. The Hydralazine suppliers may include Hydralazine API manufacturers, exporters, distributors and traders.
click here to find a list of Hydralazine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hydralazine DMF (Drug Master File) is a document detailing the whole manufacturing process of Hydralazine active pharmaceutical ingredient (API) in detail. Different forms of Hydralazine DMFs exist exist since differing nations have different regulations, such as Hydralazine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hydralazine DMF submitted to regulatory agencies in the US is known as a USDMF. Hydralazine USDMF includes data on Hydralazine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hydralazine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hydralazine suppliers with USDMF on PharmaCompass.
Hydralazine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hydralazine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hydralazine GMP manufacturer or Hydralazine GMP API supplier for your needs.
A Hydralazine CoA (Certificate of Analysis) is a formal document that attests to Hydralazine's compliance with Hydralazine specifications and serves as a tool for batch-level quality control.
Hydralazine CoA mostly includes findings from lab analyses of a specific batch. For each Hydralazine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hydralazine may be tested according to a variety of international standards, such as European Pharmacopoeia (Hydralazine EP), Hydralazine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hydralazine USP).
