24 Oct 2024
// PR NEWSWIRE
16 Oct 2024
// BUSINESSWIRE
07 Jun 2024
// Christy Santhosh REUTERS
Latest Content by PharmaCompass

About
CPhI India 2024CPhI India 2024
Industry Trade Show
Not Confirmed
26-28 November, 2024
Bio-Europe 2024Bio-Europe 2024
Industry Trade Show
Attending
04-06 November, 2024
Saudi International Ex...Saudi International Expo
Industry Trade Show
Not Confirmed
28-30 October, 2024
CONTACT DETAILS






Events
Webinars & Exhibitions
CPhI India 2024CPhI India 2024
Industry Trade Show
Not Confirmed
26-28 November, 2024
Bio-Europe 2024Bio-Europe 2024
Industry Trade Show
Attending
04-06 November, 2024
Saudi International Ex...Saudi International Expo
Industry Trade Show
Not Confirmed
28-30 October, 2024
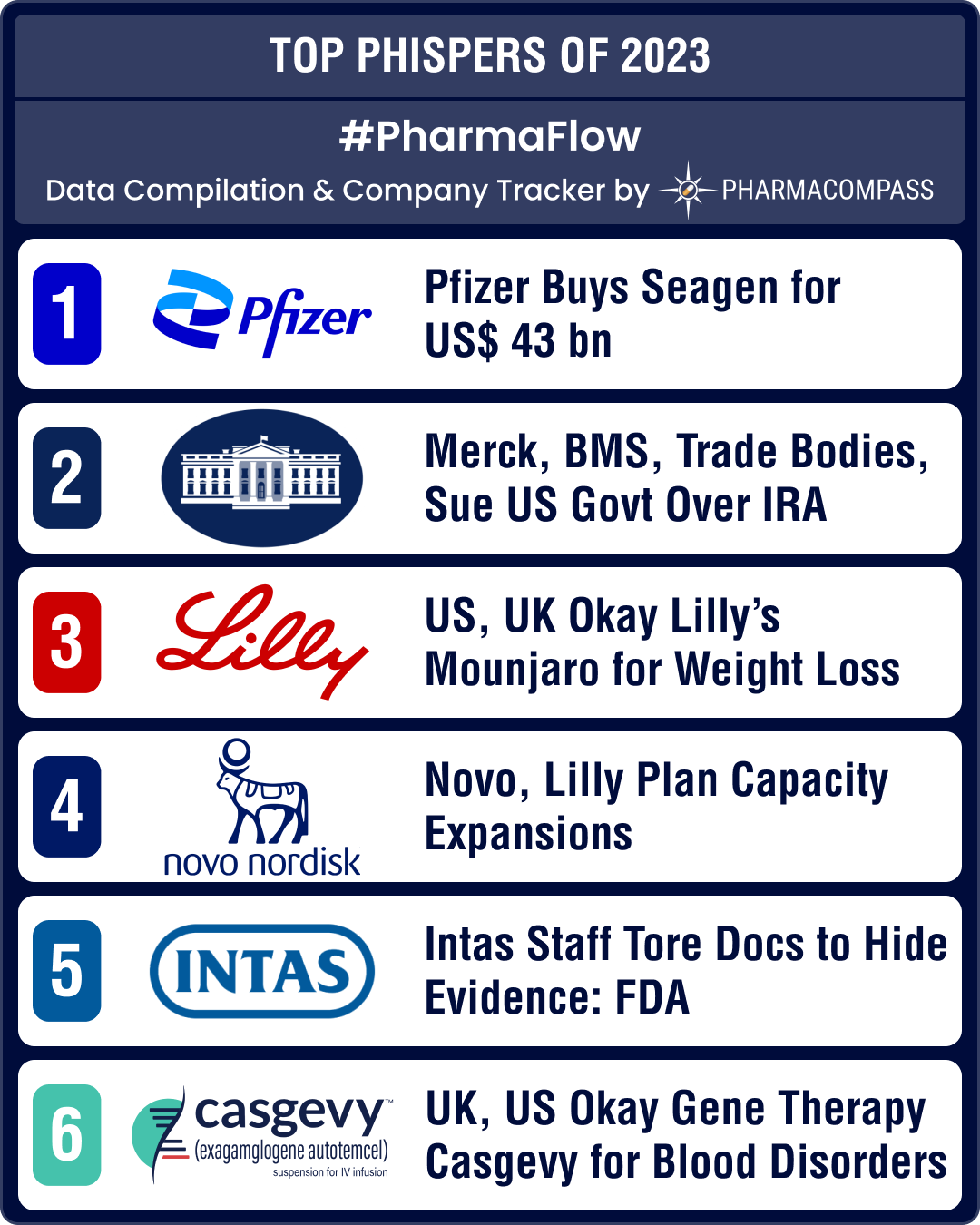
https://www.pharmacompass.com/radio-compass-blog/pfizer-s-buyout-of-seagen-drugmakers-suing-us-govt-obesity-drugs-make-it-to-top-10-phispers-of-2023

24 Oct 2024
// PR NEWSWIRE
https://www.prnewswire.com/news-releases/epivax-y-cubrc-obtienen-contrato-con-la-fda-para-el-desarrollo-de-peptidos-de-control-302286121.html

16 Oct 2024
// BUSINESSWIRE

07 Jun 2024
// Christy Santhosh REUTERS
https://www.reuters.com/business/healthcare-pharmaceuticals/abbvies-ovarian-cancer-therapy-succeeds-mid-stage-trial-2024-06-06/

14 May 2024
// PR NEWSWIRE
https://www.prnewswire.com/news-releases/genprex-doses-first-patient-in-acclaim-3-clinical-study-of-reqorsa-immunogene-therapy-in-combination-with-tecentriq-to-treat-small-cell-lung-cancer-302144415.html

02 May 2024
// BUSINESSWIRE

12 Feb 2024
// FIERCE PHARMA
https://www.fiercepharma.com/pharma/abbvie-quickly-wraps-its-101b-buyout-immunogen-another-good-sign-biopharma-ma
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
 Immunogen
Immunogen