09 Jan 2025
// PRESS RELEASE
09 Jan 2025
// PRESS RELEASE
25 Nov 2024
// PRESS RELEASE
Latest Content by PharmaCompass

 KEY PRODUCTS
KEY PRODUCTS

![]() Reset all filters
Reset all filters
01 1Sodium hyaluronate, Intrinsic viscosity 1.28 m3/kg; 1.63 m3/kg, by fermentation, for parenteral administration including intra-articular administration and for intra-ocular use
02 1Sodium hyaluronate, Intrinsic viscosity 2.93 m3/kg, by fermentation, for parenteral administration including intra-articular administration and for intra-ocular use

![]() Reset all filters
Reset all filters
01 1KEWPIE CORP. Tokyo JP
02 1KEWPIE CORPORATION Tokyo JP

![]() Reset all filters
Reset all filters
01 2Japan

![]() Reset all filters
Reset all filters
01 2Valid

Certificate Numbers : CEP 2018-037 - Rev 02
Status : Valid
Issue Date : 2025-02-21
Type : Chemical and TSE
Substance Number : 1472
Certificate Numbers : R0-CEP 2019-064 - Rev 00
Status : Valid
Issue Date : 2020-04-16
Type : Chemical and TSE
Substance Number : 1472
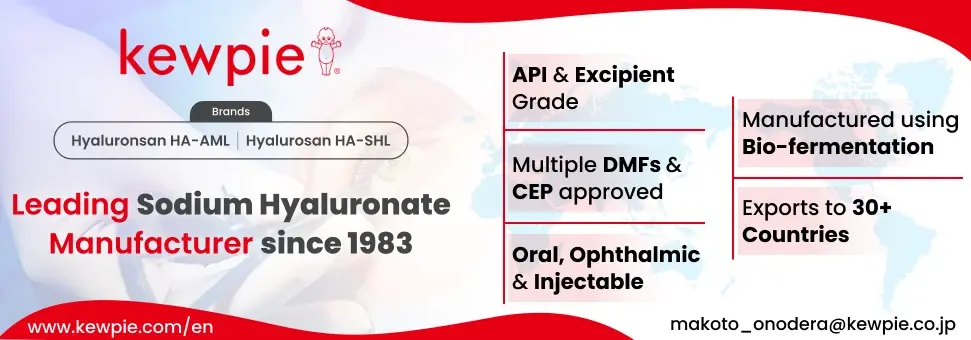
Kewpie Corporation is a supplier offers 5 products (APIs, Excipients or Intermediates).
Find a price of Sodium Hyaluronate bulk with DMF, CEP, JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk with DMF, JDMF offered by Kewpie Corporation
Find a price of Hyaluronic Acid bulk with JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk with JDMF offered by Kewpie Corporation
Find a price of Sodium Hyaluronate bulk offered by Kewpie Corporation