
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
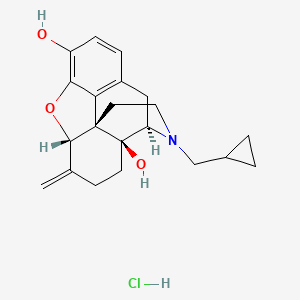

1. 6-desoxy-6-methylenenaltrexone
2. Nalmefene
3. Revex
4. Selincro
1. 58895-64-0
2. Nalmefene Hcl
3. Revex
4. Nalmefenehydrochloride
5. Jf-1 Hydrochloride
6. K7k69qc05x
7. (4r,4as,7as,12bs)-3-(cyclopropylmethyl)-7-methylidene-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,9-diol;hydrochloride
8. Unii-k7k69qc05x
9. Revex (tn)
10. 17-(cyclopropylmethyl)-4,5alpha-epoxy-6-methylenemorphinan-3,14-diol, , Hydrochloride
11. Schembl628721
12. Chembl1201152
13. Dtxsid70891705
14. Bcp08346
15. Nalmefene Hydrochloride [mi]
16. Mfcd27937056
17. Akos016340557
18. Ccg-221179
19. Hs-0037
20. Nalmefene Hydrochloride [mart.]
21. Nalmefene Hydrochloride [vandf]
22. Nalmefene Hydrochloride [who-dd]
23. Morphinan-3,14-diol, 17-(cyclopropylmethyl)-4,5-epoxy-6-methylene-, Hydrochloride, (5alpha)-
24. B7584
25. Nalmefene Hydrochloride [orange Book]
26. D02104
27. Sr-01000000007
28. Sr-01000000007-2
29. Q27282058
30. 17-cyclopropylmethyl-4,5a-epoxy-6-methylenemorphinan-3,14-diol
31. (5?)-17-(cyclopropylmethyl)-4,5-epoxy-6-methylenemorphinan-3,14-diol Hydrochloride
32. 17-cyclopropylmethyl-4,5a-epoxy-6-methylenemorphinan-3,14-diol Hydrochloride
33. (4r,4as,7as,12bs)-3-(cyclopropylmethyl)-7-methylidene-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,9-diol,hydrochloride
| Molecular Weight | 375.9 g/mol |
|---|---|
| Molecular Formula | C21H26ClNO3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 375.1601214 g/mol |
| Monoisotopic Mass | 375.1601214 g/mol |
| Topological Polar Surface Area | 52.9 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 618 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Narcotic Antagonists
Agents inhibiting the effect of narcotics on the central nervous system. (See all compounds classified as Narcotic Antagonists.)
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5484
Submission : 1984-08-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34367
Submission : 2019-12-13
Status : Active
Type : II
Date of Issue : 2021-08-27
Valid Till : 2024-06-10
Written Confirmation Number : WC-0278
Address of the Firm :

NDC Package Code : 73548-8019
Start Marketing Date : 2024-09-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : Reviewed
Rev. Date : 2019-08-25
Pay. Date : 2019-08-22
DMF Number : 15663
Submission : 2001-10-10
Status : Active
Type : II

NDC Package Code : 0406-1340
Start Marketing Date : 2025-01-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35829
Submission : 2021-11-10
Status : Active
Type : II

NDC Package Code : 0406-5690
Start Marketing Date : 2019-03-03
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-06-23
Pay. Date : 2022-04-01
DMF Number : 36751
Submission : 2022-03-21
Status : Active
Type : II

NDC Package Code : 82712-1002
Start Marketing Date : 2022-05-16
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (350g/350g)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5642
Submission : 1984-12-17
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8507
Submission : 1990-03-30
Status : Inactive
Type : II



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5484
Submission : 1984-08-01
Status : Inactive
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34367
Submission : 2019-12-13
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35829
Submission : 2021-11-10
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-08-25
Pay. Date : 2019-08-22
DMF Number : 15663
Submission : 2001-10-10
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5642
Submission : 1984-12-17
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-06-23
Pay. Date : 2022-04-01
DMF Number : 36751
Submission : 2022-03-21
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8507
Submission : 1990-03-30
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Rusan Pharma is a fully integrated global pharmaceutical company specializing in the treatment of addiction & pain management. We manufacture & market a wide range of APIs & formul...
About the Company : Guangzhou Tosun Pharmaceutical was founded in 1999, which mainly focuses on importation & exportation of Active Pharmaceutical Ingrediants, Chemical Raw Materials, Intermediate, Ex...

About the Company : OmnisMed Pharmaceuticals believes that good healthcare must be accessible to everyone; That is the core of our philosophy. We are experts in providing a wide range of high-quality ...

About the Company : We are the largest pharmaceutical company in Slovakia with site originally established in the 1940s. Our multi-purpose headquarters is easily reachable from all central European hu...

About the Company : Established in 1999, Hankang Pharmaceutical Group stands as a prominent CRO-CDMO integrated service enterprise in China. Focused on generic drugs while venturing into innovative on...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nalmefene Hydrochloride Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Zurnai
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 30, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Study on Nalmefene Injection For Opioid Overdose in ERs Concludes
Details : Nalmefene Hydrochloride Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Product Name : Zurnai
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 30, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nalmefene Hydrochloride Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Nalmefene Hydrochloride-Generic
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Accepts New Drug Application for Nalmefene Auto-Injector for Opioid Overdose Treatment
Details : Nalmefene Hydrochloride Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Product Name : Nalmefene Hydrochloride-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
August 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Nalmefene Hydrochloride Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Zurnai
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Approves First Nalmefene Hydrochloride Auto-Injector to Reverse Opioid Overdose
Details : Nalmefene Hydrochloride Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Product Name : Zurnai
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Zurnai (nalmefene hydrochloride) Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Zurnai
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Approves Zurnai™ Auto-Injector for Opioid Overdose Treatment in Adults and Children
Details : Zurnai (nalmefene hydrochloride) Injection is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.
Product Name : Zurnai
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 08, 2024

Details:
The grant will be used for the evaluation of nalmefene hydrochloride (HCl) injection, an opioid antagonist indicated for the complete or partial reversal of opioid drug effects.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: Nalmefene Hydrochloride-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Purdue Pharma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Financing March 27, 2024

Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Purdue Pharma
Deal Size : Undisclosed
Deal Type : Financing
Purdue Grants Funding for Research on Opioid Overdose Reversal with Nalmefene Injection
Details : The grant will be used for the evaluation of nalmefene hydrochloride (HCl) injection, an opioid antagonist indicated for the complete or partial reversal of opioid drug effects.
Product Name : Nalmefene Hydrochloride-Generic
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The company intends to use the net proceeds for the advancement of its phase 2 trial evaluating, TH104 (nalmefene) for the treatment of moderate-to-severe cholestatic pruritis in primary biliary cholangitis.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: TH104
Study Phase: Phase IProduct Type: Other Small Molecule
Sponsor: Thinkequity
Deal Size: $11.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering November 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Thinkequity
Deal Size : $11.0 million
Deal Type : Public Offering
Tharimmune, Inc. Announces Closing of $11 Million Public Offering
Details : The company intends to use the net proceeds for the advancement of its phase 2 trial evaluating, TH104 (nalmefene) for the treatment of moderate-to-severe cholestatic pruritis in primary biliary cholangitis.
Product Name : TH104
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
November 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TH104 is a product which has been developed by embedding drug onto a proprietary transmucosal buccal film which adheres to the inside of the mouth. It isbeing evaluated for the treatment of chronic pruritis in primary biliary cholangitis (PBC).
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: TH104
Study Phase: Phase IProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : TH104 is a product which has been developed by embedding drug onto a proprietary transmucosal buccal film which adheres to the inside of the mouth. It isbeing evaluated for the treatment of chronic pruritis in primary biliary cholangitis (PBC).
Product Name : TH104
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
November 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the agreement, Hillstream aims to acquire a clinical stage asset, AV104 (nalmefene), an opioid antagonist, but first it intends to seek approval in an orphan disease for the treatment of moderate to severe cholestatic pruritis with primary biliary cholangitis.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: AV104
Study Phase: Phase I/ Phase IIProduct Type: Other Small Molecule
Sponsor: Hillstream BioPharma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement November 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Hillstream BioPharma
Deal Size : Undisclosed
Deal Type : Agreement
Details : Through the agreement, Hillstream aims to acquire a clinical stage asset, AV104 (nalmefene), an opioid antagonist, but first it intends to seek approval in an orphan disease for the treatment of moderate to severe cholestatic pruritis with primary biliar...
Product Name : AV104
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
November 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Opvee (nalmefene) nasal spray is an opioid receptor antagonist approved by the Food and Drug Administration (FDA) to for the emergency treatment of known or suspected opioid overdose induced by natural or synthetic opioids in adults and pediatric patients.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: OPNT003
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Opvee (nalmefene) nasal spray is an opioid receptor antagonist approved by the Food and Drug Administration (FDA) to for the emergency treatment of known or suspected opioid overdose induced by natural or synthetic opioids in adults and pediatric patient...
Product Name : OPNT003
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Opvee (nalmefene) nasal spray is an opioid receptor antagonist approved by the Food and Drug Administration (FDA) to for the emergency treatment of known or suspected opioid overdose induced by natural or synthetic opioids in adults and pediatric patients.
Lead Product(s): Nalmefene Hydrochloride
Therapeutic Area: Psychiatry/Psychology Brand Name: OPNT003
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Nalmefene Hydrochloride
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Indivior Announces U.S. Food and Drug Administration Approval of OPVEE® (nalmefene) Nasal Spray, ...
Details : Opvee (nalmefene) nasal spray is an opioid receptor antagonist approved by the Food and Drug Administration (FDA) to for the emergency treatment of known or suspected opioid overdose induced by natural or synthetic opioids in adults and pediatric patient...
Product Name : OPNT003
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 23, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Selincro
Dosage Form : FILM COATED PILL
Dosage Strength : 18 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Selincro
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Selincro
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Selincro
Dosage Form : Filmtabl
Dosage Strength : 18mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Selincro
Dosage Form : Filmtabl
Dosage Strength : 18mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : REVEX
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1995-04-17
Application Number : 20459
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : REVEX
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 2MG BASE/2ML (EQ 1MG BASE/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1995-04-17
Application Number : 20459
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : OPVEE
Dosage Form : SPRAY;NASAL
Dosage Strength : EQ 2.7MG BASE/SPRAY
Packaging :
Approval Date : 2023-05-22
Application Number : 217470
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : NALMEFENE HYDROCHLORIDE
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
Packaging :
Approval Date : 2023-11-15
Application Number : 216007
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : NALMEFENE HYDROCHLORIDE
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 2MG BASE/2ML (EQ 1MG BASE/ML)
Packaging :
Approval Date : 2023-11-15
Application Number : 216007
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : REVEX
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1995-04-17
Application Number : 20459
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : REVEX
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 2MG BASE/2ML (EQ 1MG BASE/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1995-04-17
Application Number : 20459
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : OPVEE
Dosage Form : SPRAY;NASAL
Dosage Strength : EQ 2.7MG BASE/SPRAY
Approval Date : 2023-05-22
Application Number : 217470
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : NALMEFENE HYDROCHLORIDE
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 2MG BASE/2ML (EQ 1MG BASE/ML)
Approval Date : 2022-02-08
Application Number : 212955
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : ZURNAI (AUTOINJECTOR)
Dosage Form : SOLUTION;INTRAMUSCULAR, SUBCUTANEOUS
Dosage Strength : EQ 1.5MG BASE/0.5ML (EQ 1.5MG BASE/0.5ML)
Approval Date : 2024-08-07
Application Number : 218590
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : NALMEFENE HYDROCHLORIDE
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
Approval Date : 2023-11-15
Application Number : 216007
RX/OTC/DISCN : RX
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : NALMEFENE HYDROCHLORIDE
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : EQ 2MG BASE/2ML (EQ 1MG BASE/ML)
Approval Date : 2023-11-15
Application Number : 216007
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : NALMEFENE HYDROCHLORIDE
Dosage Form : SOLUTION;INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS
Dosage Strength : 2MG BASE/2ML (EQ 1MGBASE/ML)
Approval Date :
Application Number : 216007
RX/OTC/DISCN :
RLD :
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Selincro
Dosage Form : FILM COATED PILL
Dosage Strength : 18 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Selincro
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Selincro
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Selincro
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Selincro
Dosage Form : Filmtabl
Dosage Strength : 18mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Selincro
Dosage Form : Filmtabl
Dosage Strength : 18mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions

28 Oct 2024
// BUSINESSWIRE

09 Aug 2024
// FDA
https://www.fda.gov/news-events/press-announcements/fda-approves-first-nalmefene-hydrochloride-auto-injector-reverse-opioid-overdose

07 Aug 2024
// BUSINESSWIRE

08 Apr 2024
// BUSINESSWIRE

27 Mar 2024
// BUSINESSWIRE

https://www.prnewswire.com/news-releases/indivior-announces-publication-demonstrating-that-opvee-nalmefene-nasal-spray-rapidly-reverses-effects-of-opioid-induced-respiratory-depression-in-head-to-head-study-against-intranasal-naloxone-302085667.html
Global Sales Information

Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Price Per Pack (Euro) : 34.661
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Price Per Pack (Euro) : 64.581
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 18 mg
Price Per Pack (Euro) : 124.42
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Filmtabl
Dosage Strength : 18mg
Price Per Pack (Euro) : 59.76
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Dosage Form : Filmtabl
Dosage Strength : 18mg
Price Per Pack (Euro) : 165.85
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
14 Jul 2021
Reply
06 Apr 2019
Reply
13 Nov 2018

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2034-02-11
US Patent Number : 10881798
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2034-02-11

Patent Expiration Date : 2031-08-21
US Patent Number : 9364610
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-08-21

Patent Expiration Date : 2031-08-21
US Patent Number : 11446440
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-08-21

Patent Expiration Date : 2026-01-24
US Patent Number : 9533102
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-01-24

Patent Expiration Date : 2039-11-05
US Patent Number : 11857547
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2039-11-05

Patent Expiration Date : 2026-10-04
US Patent Number : 8021335
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-10-04

Patent Expiration Date : 2026-01-24
US Patent Number : 11446441
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-01-24

Patent Expiration Date : 2035-02-25
US Patent Number : 11813435
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-02-25

Patent Expiration Date : 2031-08-28
US Patent Number : 11185642
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 218590
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-08-28

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NP
Exclusivity Expiration Date : 2026-05-22
Application Number : 217470
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NP
Exclusivity Expiration Date : 2027-08-07
Application Number : 218590
Product Number : 1
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

CAS Number : 176220-84-1 (free base)
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : N0078.03

CAS Number : 42971-33-5
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : N0078.04

CAS Number :
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No : 1608

CAS Number :
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No : 1909

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
31
PharmaCompass offers a list of Nalmefene Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nalmefene Hydrochloride manufacturer or Nalmefene Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nalmefene Hydrochloride manufacturer or Nalmefene Hydrochloride supplier.
PharmaCompass also assists you with knowing the Nalmefene Hydrochloride API Price utilized in the formulation of products. Nalmefene Hydrochloride API Price is not always fixed or binding as the Nalmefene Hydrochloride Price is obtained through a variety of data sources. The Nalmefene Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Nalmefene Hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Nalmefene Hydrochloride , including repackagers and relabelers. The FDA regulates Nalmefene Hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Nalmefene Hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Nalmefene Hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Nalmefene Hydrochloride supplier is an individual or a company that provides Nalmefene Hydrochloride active pharmaceutical ingredient (API) or Nalmefene Hydrochloride finished formulations upon request. The Nalmefene Hydrochloride suppliers may include Nalmefene Hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Nalmefene Hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Nalmefene Hydrochloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Nalmefene Hydrochloride active pharmaceutical ingredient (API) in detail. Different forms of Nalmefene Hydrochloride DMFs exist exist since differing nations have different regulations, such as Nalmefene Hydrochloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Nalmefene Hydrochloride DMF submitted to regulatory agencies in the US is known as a USDMF. Nalmefene Hydrochloride USDMF includes data on Nalmefene Hydrochloride 's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Nalmefene Hydrochloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Nalmefene Hydrochloride suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Nalmefene Hydrochloride Drug Master File in Japan (Nalmefene Hydrochloride JDMF) empowers Nalmefene Hydrochloride API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Nalmefene Hydrochloride JDMF during the approval evaluation for pharmaceutical products. At the time of Nalmefene Hydrochloride JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Nalmefene Hydrochloride suppliers with JDMF on PharmaCompass.
A Nalmefene Hydrochloride written confirmation (Nalmefene Hydrochloride WC) is an official document issued by a regulatory agency to a Nalmefene Hydrochloride manufacturer, verifying that the manufacturing facility of a Nalmefene Hydrochloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Nalmefene Hydrochloride APIs or Nalmefene Hydrochloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Nalmefene Hydrochloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Nalmefene Hydrochloride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Nalmefene Hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Nalmefene Hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Nalmefene Hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Nalmefene Hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Nalmefene Hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Nalmefene Hydrochloride suppliers with NDC on PharmaCompass.
Nalmefene Hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Nalmefene Hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Nalmefene Hydrochloride GMP manufacturer or Nalmefene Hydrochloride GMP API supplier for your needs.
A Nalmefene Hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Nalmefene Hydrochloride 's compliance with Nalmefene Hydrochloride specifications and serves as a tool for batch-level quality control.
Nalmefene Hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Nalmefene Hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Nalmefene Hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Nalmefene Hydrochloride EP), Nalmefene Hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Nalmefene Hydrochloride USP).
