
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Weekly News Recap #Phispers
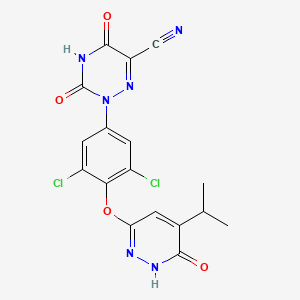

1. 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro(1,2,4)triazine-6-carbonitrile
2. Mgl-3196
1. Mgl-3196
2. 920509-32-6
3. Via-3196
4. Mgl 3196
5. 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
6. Resmetirom [usan]
7. Mgl3196
8. Re0v0t1es0
9. Chembl3261331
10. 1,2,4-triazine-6-carbonitrile, 2-(3,5-dichloro-4-((1,6-dihydro-5-(1-methylethyl)-6-oxo-3-pyridazinyl)oxy)phenyl)-2,3,4,5-tetrahydro-3,5-dioxo-
11. 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-(1,2,4)triazine-6-carbonitrile
12. 2-[3,5-dichloro-4-[(6-oxo-5-propan-2-yl-1h-pyridazin-3-yl)oxy]phenyl]-3,5-dioxo-1,2,4-triazine-6-carbonitrile
13. 2-[3,5-dichloro-4-[(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy]phenyl]-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
14. Unii-re0v0t1es0
15. Resmetirom [inn]
16. Resmetirom (usan/inn)
17. Resmetirom (mgl-3196)
18. Schembl2927241
19. Gtpl12026
20. Via3196
21. Hms3746a03
22. Amy16881
23. Bcp24624
24. Ex-a2477
25. Vlb50932
26. Bdbm50012905
27. Mfcd26142653
28. S6663
29. Who 10850
30. Zinc34842512
31. Akos032945066
32. Cs-6179
33. Db12914
34. Sb16825
35. Compound 53 [pmid: 24712661]
36. Ac-31458
37. Bs-17855
38. Hy-12216
39. Sy289460
40. Db-127167
41. C77150
42. D11602
43. Q27288071
44. 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro(1,2,4)triazine-6-carbonitrile
45. 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
46. 2-(4-(1,6-dihydro-5-isopropyl-6-oxopyridazin-3-yloxy)-3,5-dichlorophenyl)-2,3,4,5-tetrahydro-3,5-dioxo-1,2,4-triazine-6-carbonitrile
47. 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydro-pyridazin-3-yloxy)-phenyl]-3,5-dioxo-2,3,4,5-tetrahydro-[1,2,4]triazine-6-carbonitrile
| Molecular Weight | 435.2 g/mol |
|---|---|
| Molecular Formula | C17H12Cl2N6O4 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 434.0297083 g/mol |
| Monoisotopic Mass | 434.0297083 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 878 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 China's leading API and intermediate manufacturer, offering CMO/CDMO services and exports to 70+ countries, enhancing global health.
China's leading API and intermediate manufacturer, offering CMO/CDMO services and exports to 70+ countries, enhancing global health.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
 Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
Tagoor's product development expertise, backed by our comprehensive understanding of the processes, helps us offer high-quality APIs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39990
Submission : 2024-05-27
Status : Active
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40872
Submission : 2024-11-26
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 62128-0396
Start Marketing Date : 2024-03-14
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
NDC Package Code : 69988-0061
Start Marketing Date : 2023-10-04
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/1)
Marketing Category : DRUG FOR FURTHER PROCESSING

NDC Package Code : 69988-0062
Start Marketing Date : 2024-03-06
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/1)
Marketing Category : DRUG FOR FURTHER PROCESSING

NDC Package Code : 65392-3203
Start Marketing Date : 2023-11-09
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rezdiffra (resmetirom) is a once-daily, oral THR-β agonist designed to target key underlying causes of NASH. It is being studied in compensated patients with NASH cirrhosis.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Rezdiffra
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 26, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Madrigal Reports Two-Year Data Showing Rezdiffra Benefit in MASH Cirrhosis
Details : Rezdiffra (resmetirom) is a once-daily, oral THR-β agonist designed to target key underlying causes of NASH. It is being studied in compensated patients with NASH cirrhosis.
Product Name : Rezdiffra
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
February 26, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rezdiffra (resmetirom) is a once-daily, oral THR-β agonist designed to target key underlying causes of NASH. It is being studied in compensated patients with NASH cirrhosis.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Rezdiffra
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Madrigal Completes Resmetirom Trial Enrollment in Compensated NASH/MASH Cirrhosis
Details : Rezdiffra (resmetirom) is a once-daily, oral THR-β agonist designed to target key underlying causes of NASH. It is being studied in compensated patients with NASH cirrhosis.
Product Name : Rezdiffra
Product Type : Small molecule
Upfront Cash : Not Applicable
October 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PRO-140 (leronlimab) a monocloncal antibody works by blocking the activity of CCR5 receptor. It is currently in preclinical in combination with Resmetirom for the treatment of liver fibrosis.
Lead Product(s): Leronlimab,Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: PRO-140
Study Phase: PreclinicalProduct Type: Large molecule
Sponsor: SMC Laboratories
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Leronlimab,Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Preclinical
Partner/Sponsor/Collaborator : SMC Laboratories
Deal Size : Not Applicable
Deal Type : Not Applicable
CytoDyn Announces Start of Preclinical MASH Study, Results Expected in Fall 2024
Details : PRO-140 (leronlimab) a monocloncal antibody works by blocking the activity of CCR5 receptor. It is currently in preclinical in combination with Resmetirom for the treatment of liver fibrosis.
Product Name : PRO-140
Product Type : Large molecule
Upfront Cash : Not Applicable
June 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Madrigal plans to use net proceeds for commercial activities related to launching Rezdiffra (resmetirom), a liver-directed THR-β agonist for treating NASH in the U.S.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Rezdiffra
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Goldman Sachs & Co. LLC
Deal Size: $600.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering March 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Goldman Sachs & Co. LLC
Deal Size : $600.0 million
Deal Type : Public Offering
Madrigal Pharmaceuticals Announces Pricing of Upsized $600 Million Public Offering
Details : Madrigal plans to use net proceeds for commercial activities related to launching Rezdiffra (resmetirom), a liver-directed THR-β agonist for treating NASH in the U.S.
Product Name : Rezdiffra
Product Type : Small molecule
Upfront Cash : Undisclosed
March 18, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Rezdiffra (resmetirom) is a once-daily oral THR-β agonist approved for treating noncirrhotic nonalcoholic steatohepatitis (NASH) with moderate to advanced liver fibrosis.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: Rezdiffra
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Madrigal's Rezdiffra Approved by FDA for NASH with Liver Fibrosis
Details : Rezdiffra (resmetirom) is a once-daily oral THR-β agonist approved for treating noncirrhotic nonalcoholic steatohepatitis (NASH) with moderate to advanced liver fibrosis.
Product Name : Rezdiffra
Product Type : Small molecule
Upfront Cash : Not Applicable
March 14, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MGL-3196 (Resmetirom) is a once-daily, oral, liver-directed THR-β agonist , which is being evaluated for the treatment of NASH/MASH with Liver Fibrosis.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: MGL-3196
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Madrigal Pharmaceuticals Announces EMA Validation of Resmetirom Application for NASH
Details : MGL-3196 (Resmetirom) is a once-daily, oral, liver-directed THR-β agonist , which is being evaluated for the treatment of NASH/MASH with Liver Fibrosis.
Product Name : MGL-3196
Product Type : Small molecule
Upfront Cash : Not Applicable
March 05, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Madrigal will use the proceeds for its clinical and commercial activities in preparation for a potential launch of MGL-3196 (resmetirom), a once daily, oral, thyroid hormone receptor-beta selective agonist designed to target key underlying causes of NASH in the liver.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: MGL-3196
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Goldman Sachs & Co. LLC
Deal Size: $500.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Goldman Sachs & Co. LLC
Deal Size : $500.0 million
Deal Type : Public Offering
Madrigal Pharmaceuticals Announces Pricing of $500 Million Public Offering
Details : Madrigal will use the proceeds for its clinical and commercial activities in preparation for a potential launch of MGL-3196 (resmetirom), a once daily, oral, thyroid hormone receptor-beta selective agonist designed to target key underlying causes of NASH...
Product Name : MGL-3196
Product Type : Small molecule
Upfront Cash : Undisclosed
September 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MGL-3196 (resmetirom) is a once daily, oral, thyroid hormone receptor (THR)-beta selective agonist designed to target key underlying causes of NASH in the liver.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: MGL-3196
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : MGL-3196 (resmetirom) is a once daily, oral, thyroid hormone receptor (THR)-beta selective agonist designed to target key underlying causes of NASH in the liver.
Product Name : MGL-3196
Product Type : Small molecule
Upfront Cash : Not Applicable
September 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MGL-3196 (resmetirom), is a once daily, oral, liver-directed selective thyroid hormone receptor (THR)-β selective agonist designed to target key underlying causes of NASH in the liver.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: MGL-3196
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable July 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : MGL-3196 (resmetirom), is a once daily, oral, liver-directed selective thyroid hormone receptor (THR)-β selective agonist designed to target key underlying causes of NASH in the liver.
Product Name : MGL-3196
Product Type : Small molecule
Upfront Cash : Not Applicable
July 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MGL-3196 (resmetirom), is a once daily, oral, liver-directed selective thyroid hormone receptor (THR)-β selective agonist designed to target key underlying causes of NASH in the liver.
Lead Product(s): Resmetirom
Therapeutic Area: Hepatology (Liver, Pancreatic, Gall Bladder) Brand Name: MGL-3196
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 18, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Resmetirom
Therapeutic Area : Hepatology (Liver, Pancreatic, Gall Bladder)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : MGL-3196 (resmetirom), is a once daily, oral, liver-directed selective thyroid hormone receptor (THR)-β selective agonist designed to target key underlying causes of NASH in the liver.
Product Name : MGL-3196
Product Type : Small molecule
Upfront Cash : Not Applicable
April 18, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]6-(4-amino-2,6-dichlorophenoxy)- 4-isopropylpyrida...
CAS Number : 920509-28-0
End Use API : Resmetirom
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
3,6-dichloro-4-isopropylpyridazine
CAS Number : 107228-51-3
End Use API : Resmetirom
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
4-(Propan-2-yl)pyridazine-3,6-diol
CAS Number : 1903632-97-2
End Use API : Resmetirom
About The Company : Shandong Chenghui Shuangda Pharmaceutical Co., Ltd. is specialized in R&D and production of APIs and advanced intermediates. With 22 years of production experie...
3,6-Dichloro-4-isopropylpyridazine
CAS Number : 107228-51-3
End Use API : Resmetirom
About The Company : Royal pharma was started in 2007 – it’s a small scale Advanced Intermediates Manufacturing company. Facilities are in accordance to GMP standards. Currently...

6-(4-Amino-2,6-dichlorophenoxy)-4-isopropylpyridaz...
CAS Number : 920509-28-0
End Use API : Resmetirom
About The Company : Royal pharma was started in 2007 – it’s a small scale Advanced Intermediates Manufacturing company. Facilities are in accordance to GMP standards. Currently...

3,6-Dichloro-4-isopropylpyridazine transportation
CAS Number : 107228-51-3
End Use API : Resmetirom
About The Company : SS Pharmaceutical develops and manufactures active pharmaceutical ingredients (APIs), registered pharma intermediates, fine chemicals and pyridine derivatives f...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : REZDIFFRA
Dosage Form : TABLET;ORAL
Dosage Strength : 60MG
Packaging :
Approval Date : 2024-03-14
Application Number : 217785
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : REZDIFFRA
Dosage Form : TABLET;ORAL
Dosage Strength : 80MG
Packaging :
Approval Date : 2024-03-14
Application Number : 217785
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : REZDIFFRA
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG
Packaging :
Approval Date : 2024-03-14
Application Number : 217785
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : REZDIFFRA
Dosage Form : TABLET;ORAL
Dosage Strength : 60MG
Approval Date : 2024-03-14
Application Number : 217785
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : REZDIFFRA
Dosage Form : TABLET;ORAL
Dosage Strength : 80MG
Approval Date : 2024-03-14
Application Number : 217785
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : REZDIFFRA
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG
Approval Date : 2024-03-14
Application Number : 217785
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
25 Dec 2024
Reply
11 Oct 2024
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2029-03-14
Application Number : 217785
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2029-03-14
Application Number : 217785
Product Number : 3
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2029-03-14
Application Number : 217785
Product Number : 2
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
57
PharmaCompass offers a list of Resmetirom API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Resmetirom manufacturer or Resmetirom supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Resmetirom manufacturer or Resmetirom supplier.
PharmaCompass also assists you with knowing the Resmetirom API Price utilized in the formulation of products. Resmetirom API Price is not always fixed or binding as the Resmetirom Price is obtained through a variety of data sources. The Resmetirom Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Resmetirom manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Resmetirom, including repackagers and relabelers. The FDA regulates Resmetirom manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Resmetirom API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Resmetirom manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Resmetirom supplier is an individual or a company that provides Resmetirom active pharmaceutical ingredient (API) or Resmetirom finished formulations upon request. The Resmetirom suppliers may include Resmetirom API manufacturers, exporters, distributors and traders.
click here to find a list of Resmetirom suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Resmetirom DMF (Drug Master File) is a document detailing the whole manufacturing process of Resmetirom active pharmaceutical ingredient (API) in detail. Different forms of Resmetirom DMFs exist exist since differing nations have different regulations, such as Resmetirom USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Resmetirom DMF submitted to regulatory agencies in the US is known as a USDMF. Resmetirom USDMF includes data on Resmetirom's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Resmetirom USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Resmetirom suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Resmetirom as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Resmetirom API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Resmetirom as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Resmetirom and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Resmetirom NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Resmetirom suppliers with NDC on PharmaCompass.
Resmetirom Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Resmetirom GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Resmetirom GMP manufacturer or Resmetirom GMP API supplier for your needs.
A Resmetirom CoA (Certificate of Analysis) is a formal document that attests to Resmetirom's compliance with Resmetirom specifications and serves as a tool for batch-level quality control.
Resmetirom CoA mostly includes findings from lab analyses of a specific batch. For each Resmetirom CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Resmetirom may be tested according to a variety of international standards, such as European Pharmacopoeia (Resmetirom EP), Resmetirom JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Resmetirom USP).
