
1. 2-hydroheptafluoropropane
2. 2h-heptafluoropropane
3. Apaflurane
4. Hfa 227
5. Hfa-227
6. Hydrofluoroalkane
1. 431-89-0
2. Apaflurane
3. 2h-heptafluoropropane
4. Hfc-227ea
5. 2-hydroheptafluoropropane
6. Solkane 227
7. Propane, 1,1,1,2,3,3,3-heptafluoro-
8. Hfa-227
9. Hfc 227
10. Hfa 227ea
11. Hfc 227ea
12. R40p36gdk6
13. Hydrofluorocarbon 227ea
14. 1,1,1,2,3,3,3-heptafluoropropane (fc 227ea)
15. R-227
16. Khladon 227
17. 2-hydroperfluoropropane
18. Apaflurane [inn:ban]
19. 2h-perfluoropropane
20. Ccris 7786
21. Hfa 227
22. Einecs 207-079-2
23. Fm 200
24. Unii-r40p36gdk6
25. Hsdb 7830
26. Hfc-227
27. Apaflurane [ii]
28. Apaflurane (inn/ban)
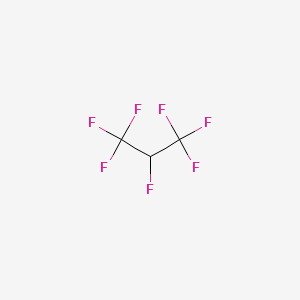
29. Cf3chfcf3
30. Apaflurane [inn]
31. Ec 207-079-2
32. Apaflurane [mart.]
33. Schembl19860
34. Chembl2104472
35. Dtxsid4042048
36. Zinc8214485
37. Mfcd00043834
38. 1,1,1,2,3,3,3 Heptafluoropropane
39. Akos006229361
40. 1,1,1,2,3,3,3-heptafluoro-propane
41. Hydrofluorocarbon 227ea [inci]
42. Db-051054
43. 1,1,1,2,3,3,3-heptakis(fluoranyl)propane
44. 2-propanyl, 1,1,1,2,3,3,3-heptafluoro-
45. D10216
46. A826208
47. Q2683759
| Molecular Weight | 170.03 g/mol |
|---|---|
| Molecular Formula | C3HF7 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 0 |
| Exact Mass | 169.99664717 g/mol |
| Monoisotopic Mass | 169.99664717 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 93 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Oral absorption is rapid but less complete than pulmonary absorption. Skin absorption is insignificant, except in patients with skin breakdown (e.g., burns, ulcers, severe ichthyosis). Increased metabolic rate can lead to greater inhalational absorption as well. Peak blood levels occur soon after inhalation but occur in 1 to 2 hours after oral administration. The chemicals distribute to tissues with high blood flow (e.g., brain, heart, liver, kidney) and then to adipose tissue, where the highest chemical concentrations are typically found. Halogenated hydrocarbons are metabolized in the liver by cytochrome P-450 oxidation. Partial glutathione conjugation may occur. Halogenated solvents can be excreted unchanged through the lungs. Elimination half-lives can be increased because of either prolonged exposure or hepatic dysfunction. Prolonged exposure allows more chemical to be stored in the adipose tissue, which serves as a source of continued release. /Halogenated Hydrocarbons - Halogenated Solvents/
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1339
... The safety and pharmacokinetics of HFC 134a (1,1,1,2-tetrafluoroethane) and HFC 227 (1,1,1,2,3,3, 3-heptafluoropropane) were assessed in two separate double-blind studies. Each HFC (hydrofluorocarbon) was administered via whole-body exposure as a vapor to eight (four male and four female) healthy volunteers. Volunteers were exposed, once weekly for 1 hr, first to air and then to ascending concentrations of HFC (1000, 2000, 4000, and 8000 ppm), interspersed with a second air exposure and two CFC 12 (dichlorodifluoromethane) exposures (1000 and 4000 ppm). HFC 134a, HFC 227, and CFC 12 blood concentrations increased rapidly and in an exposure-concentration-dependent manner, although not strictly proportionally, and approached steady state. Maximum blood concentrations (C(max)) tended to be higher in males than females; in the HFC 227 study, these were statistically significantly (P < 0. 05) higher in males for each HFC 227 and CFC 12 exposure level. ... In the HFC 227 study, t(1/2)alpha (alpha elimination half-life) for both CFC 12 and HFC 227, at each exposure level, was short (< 9 min) and tended to be lower in males than females. For CFC 12 mean t(1/2)beta (beta elimination half-life) ranged from 23 to 43 min and for HFC 227 the mean range was 19-92 min. The values tended to be lower for females than males for HFC 227. For both CFC 12 and HFC 227, mean residence time (MRT) was statistically significantly lower (P < 0.05) in males than females and independent of exposure concentration. For CFC 12, MRT was a mean of 37 and 45 min for males and females, respectively, and for HFC 227 MRT was a mean of 36 and 42 min, respectively...
PMID:11029265 Emmen HH et al; Regul Toxicol Pharmacol 32 (1): 22-35 (2000)
The biotransformation of the aerosol propellant 1,1,1,2,3,3,3-heptafluoropropane (HFA-227) was investigated in rats in vivo and in rat and human liver microsomes. In the urine of rats exposed to 5000 ppm HFA-227 for 6 hr, very small amounts of hexafluoroacetone trihydrate were identified as an HFA-227 metabolite by 19F-NMR. Fluoride concentrations in the urine samples (0-48 hr after the end of the exposure) from exposed animals were not significantly different from those found in samples from nonexposed rats. In rat and human liver microsomes, fluoride and hexafluoroacetone trihydrate formation from HFA-227 was detected in very low levels only in liver microsomes from pyridine-treated rats and in two of eight human liver microsome samples, which exhibited the highest cytochrome P4502E1 activities. Because some aldehydes may covalently bind to proteins and the formation of fluorinated protein adducts has been implicated in immune-mediated hepatitis induced by halothane, the binding of hexafluoroacetone trihydrate to proteins was also investigated. Hexafluoroacetone trihydrate also gave only a very small resonance in fluorine NMR experiments when binding to human serum albumin was studied in comparison with the acylating agent S-ethyltrifluoroacetate. Moreover, no fluorine-containing products were formed by the reaction of hexafluoroacetone trihydrate with N alpha-acetyl-L-lysine, and hexafluoroacetone trihydrate was not metabolized to fluorine-containing metabolites or inorganic fluoride in rats. Comparative studies in human liver microsomes demonstrated that a halothane metabolite may covalently bind to proteins; in contrast, metabolism and covalent binding of HFA-227 could not be demonstrated. In summary, these data indicate that HFA-227 is biotransformed at very low rates to hexafluoroacetone trihydrate but irreversible binding of hexafluoroacetone trihydrate cannot be demonstrated, even with the application of very sensitive methods, and is considered unlikely, based on the combination of the results obtained.
PMID:8869827 Koster U et al; Drug Metab Dispos 24 (8): 906-10 (1996)
HFA 227 showed a biphasic elimination from the body after exposure (mean T1/2: approx. 6.5 minutes and 44.5 minutes).
Solvay; Solkane 227 Pharma and Solkane 134a Pharma: HFA Propellants for Medical Use, p.29. Available from, as of March 3, 2016: https://www.solvay.us/en/