
1. Fc-134a
2. Gr 106642x
3. Hfa 134a
4. Hfa-134a
5. Hfc 134a
6. Hfc-134a
7. Hfc134a
8. Hydrofluoroalkane-134a
9. Norflurane
10. R 134a
11. R-134a
1. Norflurane
2. 811-97-2
3. Hfc-134a
4. Tetrafluoroethane
5. 1,2,2,2-tetrafluoroethane
6. R-134a
7. Hfa 134a
8. Ethane, 1,1,1,2-tetrafluoro-
9. Hfa-134a
10. R 134a
11. Genetron 134a
12. Ethane, Tetrafluoro-
13. 29759-38-4
14. Dymel 134a
15. Suva 134a
16. Solkane 134a
17. Forane 134a
18. Freon 134a
19. Hfa-134-a
20. Hydrofluorocarbon 134a
21. Dh9e53k1y8
22. Refrigerant R134a
23. Khladon 134a
24. 1,1,1,2-tetrafluoroethane 100 Microg/ml In Methanol
25. Arcton 134a
26. Hfc 134a
27. Fron 134a
28. Norflurano
29. Norfluranum
30. Norfluran
31. Norfluranum [inn-latin]
32. Norflurano [inn-spanish]
33. 1,1,1,2-tetrafluorethan
34. Hcfc 134a
35. Norflurane [usan:inn:ban]
36. Fc 134a
37. Tg 134a
38. Ccris 7214
39. F 134a
40. Hsdb 6756
41. Einecs 212-377-0
42. Un3159
43. Unii-dh9e53k1y8
44. R134a
45. Norflurane [ii]
46. Norflurane [inn]
47. Norflurane (usan/inn)
48. Norflurane [hsdb]
49. Norflurane [usan]
50. Cf3ch2f
51. Ec 212-377-0
52. Norflurane [mart.]
53. Schembl9499
54. Norflurane [who-dd]
55. 1,1,1,2 Tetrafluoroethane
56. Hfa-134a Propellant
57. 1,1,1,2-tetrafluoro-ethane
58. Schembl1789650
59. Chembl2104432
60. Dtxsid1021324
61. Hfc-134a [mi]
62. Tetrafluoroethane [vandf]
63. Norflurane [ep Monograph]
64. Amy25787
65. Zinc8214630
66. 1,1,1,2-tetrakis(fluoranyl)ethane
67. Mfcd00066608
68. Akos015853137
69. Db13116
70. Hydrofluorocarbon 134a [inci]
71. 1,1,1,2-tetrafluoroethane, >=99%
72. 1,1,1,2-tetrafluoroethane (hfc-134a)
73. Db-075746
74. Ft-0605908
75. T1476
76. D05208
77. A840070
78. Q423029
79. 1,1,1,2-tetrafluoroethane Or Refrigerant Gas R 134a
80. 1,1,1,2-tetrafluoroethane Or Refrigerant Gas R 134a [un3159] [nonflammable Liquid]
| Molecular Weight | 102.03 g/mol |
|---|---|
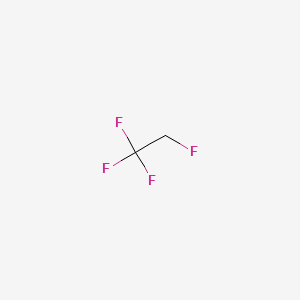
| Molecular Formula | C2H2F4 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 102.00926271 g/mol |
| Monoisotopic Mass | 102.00926271 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 35.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. 1,1,1,2-Tetrafluoroethane is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=1%2C1%2C1%2C2-TETRAFLUOROETHANE&Search=Search
Anesthetics
Agents capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general ANESTHESIA, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. (See all compounds classified as Anesthetics.)
Aerosol Propellants
Compressed gases or vapors in a container which, upon release of pressure and expansion through a valve, carry another substance from the container. They are used for cosmetics, household cleaners, and so on. Examples are BUTANES; CARBON DIOXIDE; FLUOROCARBONS; NITROGEN; and PROPANE. (McGraw-Hill Dictionary of Scientific and Technical Terms, 4th ed) (See all compounds classified as Aerosol Propellants.)
The safety and pharmacokinetics of HFC 134a and HFC 227 were assessed in two separate double-blind studies. Each HFC (hydrofluorocarbon) was administered via whole-body exposure as a vapor to eight (four male and four female) healthy volunteers. Volunteers were exposed, once weekly for 1 hr, first to air and then to ascending concentrations of HFC (1000, 2000, 4000, and 8000 parts per million (ppm)), interspersed with a second air exposure and two CFC 12 (dichlorodifluoromethane) exposures (1000 and 4000 ppm). Comparison of either HFC 134a or HFC 227 to CFC 12 or air gave no clinically significant results for any of the measured laboratory parameters. There were no notable adverse events, there was no evidence of effects on the central nervous system, and there were no symptoms of upper respiratory tract irritation. HFC 134a, HFC 227, and CFC 12 blood concentrations increased rapidly and in an exposure-concentration-dependent manner, although not strictly proportionally, and approached steady state. Maximum blood concentrations (C(max)) tended to be higher in males than females. ... In the HFC 134a study, the gender difference in C(max) was only statistically significant (P < 0.05) for CFC 12 at 4000 ppm and HFC 134a at 8000 ppm. Following the end of exposure, blood concentrations declined rapidly, predominantly biphasically and independent of exposure concentration. For the HFC 134a study, the t(1/2)alpha (alpha elimination half-life) was short for both CFC 12 and HFC 134a (<11 min). The t(1/2)beta (beta elimination half-life) across all exposure concentrations was a mean of 36 and 42 min for CFC 12 and HFC 134a, respectively. Mean residence time (MRT) was an overall mean of 42 and 44 min for CFC 12 and HFC 134a, respectively. ... Exposure of healthy volunteers to exposure levels up to 8000 ppm HFC 134a, 8000 ppm HFC 227, and 4000 ppm CFC 12 did not result in any adverse effects on pulse, blood pressure, electrocardiogram, or lung function.
PMID:11029265 Emmen HH et al; Regul Toxicol Pharmacol 32 (1): 22-35 (2000)
Six male and six female Sprague-Dawley rats were ventilated head-only for 1 hr on a 15% atmosphere of 1,1,1,2-tetrafluoroethane (HFA-134a) in air in a magnetic resonance imaging spectrometer. Results from these dynamic 19F NMR studies suggest that a steady-state in vivo concentration of HFA-134a was approached at approximately 25 min into the exposure. Quantitative integration analysis using an external standard estimated this plateau to be 58.3 +/- 11.9 mg of absorbed HFA-134a per rat. The HFA-134a 19F NMR signal disappeared rapidly following removal of the test atmosphere, with an elimination half-life of 4.6 +/- 0.6 min in the male rats and 4.9 +/- 1.5 min in the female rats. The data suggest that there was no statistical difference between the sexes in amount absorbed or in elimination half-lives.
PMID:7760708 Finch JR et al; Magn Reson Med 33 (3): 409-13 (1995)
HFA134a (1,1,1,2-tetrafluoroethane) is a nonozone-depleting candidate to replace the chlorofluorocarbons used as propellants in metered-dose inhalers (MDIs) for pharmaceuticals that are widely used in the treatment of respiratory tract disease. As a means for ensuring the safety of such a compound for human use, it is necessary to establish that there is no excessive or unexpected accumulation in the body and in selected regions. A sensitive whole-body gamma-counting technique has been used with 18F-labeled HFA134a to measure the whole-body and regional absorption, distribution, and retention of HFA134a after administration in humans by single-breath inhalation. In seven healthy subjects, labeled HFA134a was rapidly eliminated by ventilation during the first few minutes, with an average of 9.6% of the radioactivity retained in the body at 5 min. This radioactivity cleared with an apparent terminal half-life of 1.5-4.2 hr to leave, on average, < 1% of the administered dose (< 750 micrograms, approximately 0.2 microCi) retained in the body at 5.8 hr. Disposition of radioactivity was independent of the position of label. Thus, there was no evidence of any significant degradative metabolism. On average, only 0.0056% of the administered dose appeared in the urine within the first 2 hr. Later samples contained no significant radioactivity. Inhaled HFA134a first distributed to all regions of the body and then cleared without evident accumulation in any specific region.
PMID:7493550 Pike VW et al; Drug Metab Dispos 23 (8): 832-9 (1995)
The safety, tolerability and pharmacokinetics of the chlorine-free propellant HFA134a were assessed in healthy subjects after single and repeat doses. Absorption and disposition were investigated in healthy subjects and severe chronic obstructive pulmonary disease (COPD) patients using labelled HFA134a. There were no clinically significant changes in vital signs, ECG, pulmonary function tests and laboratory parameters measured. No serious adverse events were reported. In both subjects and patients HFA134a was mainly eliminated by exhalation within the first few minutes after administration and was distributed throughout the body with no obvious accumulation in any specific region. HFA134a was rapidly absorbed after inhalation with dose-related blood concentrations which declined rapidly after dosing (t1/2 = 31 min). Metabolism was not a significant route of elimination of HFA134a. Studies were also performed with salmeterol and salbutamol MDIs reformulated with HFA134a. The results showed that these MDIs were safe and well tolerated in healthy subjects and gave a similar pharmacodynamic response to the current MDIs.
PMID:10150494 Ventresca GP; J Aerosol Med 8 Suppl 1: S35-9 (1995)
For more Absorption, Distribution and Excretion (Complete) data for 1,1,1,2-TETRAFLUOROETHANE (8 total), please visit the HSDB record page.
The metabolic fate and disposition of [U-(14)C]-1,1,1,2-tetrafluoroethane ([U-(14)C]-HFC134a) has been determined in the male and female rat following a 1 hr single exposure by inhalation to atmospheres of 10,000 ppm. Of the inhaled dose, approx. 1% was recovered in urine, feces and expired air postexposure indicating that absorption of this fluorocarbon across the lung is poor. Of this 1%, approx, two-thirds were exhaled within 1 hr of the cessation of exposure as unchanged HFC134a. The remaining radioactivity was exhaled as [(14)C]-carbon dioxide or excreted in urine and feces as trifluoroacetic acid. Carbon dioxide was the major metabolite of HFC134a accounting for 0.22 and 0.27% of the inhaled dose in the male and female rat, respectively. Urinary excretion accounted for 0.09% of the dose and fecal excretion 0.04% of the dose by both sexes. Total metabolism measured as the sum of the radioactivities in urine, feces and as carbon dioxide amounted to 0.34 and 0.40% of the inhaled dose in male and female, respectively. There were no major sex differences in the rates, routes or amounts of radiolabel excreted. Analysis of a range of tissues at 5 days postexposure showed a relatively uniform distribution of radioactivity. There was no evidence for a specific uptake of HFC134a or a metabolite into any organ or tissue analyzed, including fat.
PMID:8237055 Ellis MK et al; Xenobiotica 23 (7): 719-29 (1993)
The chlorofluorocarbon substitute 1,1,1,2-tetrafluoroethane is subject to metabolism by cytochrome p450 in hepatic microsomes from rat, rabbit, and human. In rat and rabbit, the p450 form 2E1 is a predominant low KM, high rate catalyst of 1,1,1,2-tetrafluoroethane biotranformation and is prominently involved in the metabolism of other tetrahaloalkanes of greater toxicity than 1,1,1,2-tetrafluoroethane (eg, 1,2-dichloro-1,1-difluoroethane). In this study, it was determined that the human ortholog of p450 2E1 plays a role of similar importance in the metabolism of 1,1,1,2-tetrafluoroethane. In human hepatic microsomes from 12 individuals, preparations from subjects with relatively high p450 2E1 levels were shown to metabolize 1,1,1,2-tetrafluoroethane at rates 5- to 10-fold greater than microsomes of individuals with lower levels of this enzyme; the increased rate of metabolism of 1,1,1,2-tetrafluoroethane was specifically linked to increased expression of p450 2E1. The primary evidence for the conclusion is drawn from studies using mechanism based inactivation of p450 2E1 by diethyldithiocarbamate, competitive inhibition of 1,1,1,2-tetrafluoroethane oxidation by p-nitrophenol (a high affinity substrate for p450 2E1), strong positive correlations of rates of 1,1,1,2-tetrafluoroethane defluorination with p-nitrophenol hydroxylation in the study population, and correlation of p450 2E1 levels with rates of halocarbon oxidation. Thus, our findings support the conclusion that human metabolism of 1,1,1,2-tetrafluoroethane is qualitatively similar to that of the species (rat and rabbit) used for toxicological assessment of this halocarbon. Although hazard from 1,1,1,2-tetrafluoroethane exposure is not anticipated in most humans (based on toxicological evaluation in laboratory animals), our results suggest that 1,1,1,2-tetrafluoroethane exposure should be minimized for individuals with chemical exposure histories commensurate with elevation of p450 2E1 (ie, frequent contact with agents such as ethanol, trichloroethylene, or pyridine). Furthermore, these findings suggest that toxicity assessment of certain other haloethanes currently under consideration as replacements for chlorofluorocarbons should be considered in animals with elevated p450 2E1.
PMID:1356728 Surbrook S E Jr, Olson MJ; Drug Metab Dispos 20 (4): 518-24 (1992)
1,1,1,2-Tetrafluoroethane, which lacks ozone depleting potential, has been selected as a replacement refrigerant for dichlorodifluoromethane in air conditioning and chiller applications, and as a propellant for pharmaceutical aerosols. A variety of paradigms using rats and rabbits have shown that 1,1,1,2-tetrafluoroethane has very little toxic potential. To strengthen the prediction of human hazard associated with 1,1,1,2-tetrafluoroethane exposure, the rate of metabolism of this halocarbon by human hepatic microsomes was evaluated relative to similar tissue preparations derived from rats and rabbits. Human microsomes defluorinated 1,1,1,2-tetrafluoroethane in a cytochrome p450 catalyzed reaction, common also to rat and rabbit. In absolute terms, the maximal rate of 1,1,1,2-tetrafluoroethane metabolism by human microsomes was very low, showed little interindividual variation among the samples evaluated (1.3 : 0.3 nmol fluoride ions/mg protein/15 min, x : standard deviation, n = 10), and did not exceed that in rat or rabbit liver microsomes. These findings support the argument that for characterization of 1,1,1,2-tetrafluoroethane toxicity, especially that which may be mediated by products of halocarbon metabolism, laboratory animals are an adequate surrogate for humans.
Olson MJ, Surbrook S E Jr; Toxicol Lett (AMST) 59 (1-3): 89-100 (1991)
1,1,1,2-Tetrafluoroethane (R-134a), a nonozone-depleting alternative air-conditioning refrigerant and propellant for pharmaceutical preparations, is oxidatively defluorinated by rat hepatic microsomes. In this report we show that induction of cytochrome P-450IIE1 in rats, by pyridine administration, resulted in an 8-fold increase in the rate of R-134a metabolism by hepatic microsomes (Vmax 47 vs. 6 nmol F-/mg microsomal protein/15 min). Furthermore, when data were normalized for P-450 content, a 4-fold increase in R-134a metabolism was noted for IIE1-enriched microsome preparations. In contrast, phenobarbital and Aroclor 1254 decreased the specific activity of hepatic microsomes for this function. The microsomal content of P-450IIE1, as evaluated by Western blot, was elevated significantly only in microsomes from pyridine-treated rats. p-Nitrophenol and aniline, which are metabolized at high rates by rat P-450IIE1, decreased the rate of R-134a defluorination by hepatic microsomes; Dixon plot analysis indicated competitive inhibition with a Ki of 36 microM p-nitrophenol or 115 microM aniline. Pyridine also potently induced defluorination of R-134a catalyzed by rabbit liver microsomes. Studies with individual P-450 isozymes purified from rabbit liver showed that the phenobarbital- and polycyclic hydrocarbon-induced isozymes (IIB1 and IA2) defluorinated R-134a at negligible rates (1.9 and 0.4 nmol F-/nmol P-450/60 min, respectively). In contrast, P-450IIE1 catalyzed defluorination of R-134a at a relatively high rate (16.2 nmol F-/nmol P-450/60 min); isozyme IA1, which also is induced by nitrogen-containing heterocycles such as pyridine, was somewhat active (5.3 nmol F-/nmol P-450/60 min).
PMID:1676626 Olson MJ et al; Drug Metab Dispos 19 (2): 298-303 (1991)
The metabolism of 1,1,1,2-tetrafluoroethane by hepatocytes was investigated. Liver cells were isolated from male Fischer 344 rats and exposed to atmospheres containing 1,1,1,2-tetrafluoroethane and/or halothane and analyzed for fluoride. Fluoride was detected after exposure of hepatocytes to 25% 1,1,1,2-tetrafluoroethane, and the amount increased with the number of cells and with increasing 1,1,1,2-tetrafluoroethane concn. A nonlinear relationship was seen between 1,1,1,2-tetrafluoroethane concn and fluoride, indicating probable substrate saturation. When hepatocytes were incubated with 25% 1,1,1,2-tetrafluoroethane and halothane, there was a reduction in fluoride production that was related to the concn of halothane. Hepatocytes from phenobarbital treated animals produced as much fluoride as untreated animals in the presence of 12.5% or less 1,1,1,2-tetrafluoroethane, however at a concn of 25% or more 1,1,1,2-tetrafluoroethane, phenobarbital treated cells produced more fluoride than untreated cells. It was concluded that 1,1,1,2-tetrafluoroethane can be metabolized by liver cells, and may involve cytochrome p450.
Olson MJ et al; Biochem Biophys Res Communic 166 (3): 1390-7 (1990)
... Ten male volunteers were exposed to 500 ppm HFC-134a (2 hr, 50 W exercise). ... The post-exposure urinary excretion was 0.002% of the inhaled amount, and the half-time was 58 min (pooled data). ... The late decay in blood was consistent with a wash-out of HFC-134a from fat tissues, with a half-time of 114+/-21 min. ...
PMID:17030466 Gunnare S et al; Toxicol Lett 167 (1): 54-65 (2006)
... HFA134a was rapidly absorbed after inhalation with dose-related blood concentrations which declined rapidly after dosing (t1/2 = 31 min). ...
PMID:10150494 Ventresca GP; J Aerosol Med 8 Suppl 1: S35-9 (1995)
Six male and six female Sprague-Dawley rats were ventilated head-only for 1 hr on a 15% atmosphere of 1,1,1,2-tetrafluoroethane (HFA-134a) in air in a magnetic resonance imaging spectrometer. ... The HFA-134a 19F NMR signal disappeared rapidly following removal of the test atmosphere, with an elimination half-life of 4.6 +/- 0.6 min in the male rats and 4.9 +/- 1.5 min in the female rats. ...
PMID:7760708 Finch JR et al; Magn Reson Med 33 (3): 409-13 (1995)
The safety and pharmacokinetics of HFC 134a and HFC 227 were assessed in two separate double-blind studies. Each HFC (hydrofluorocarbon) was administered via whole-body exposure as a vapor to eight (four male and four female) healthy volunteers. Volunteers were exposed, once weekly for 1 hr, first to air and then to ascending concentrations of HFC (1000, 2000, 4000, and 8000 parts per million (ppm)), interspersed with a second air exposure and two CFC 12 (dichlorodifluoromethane) exposures (1000 and 4000 ppm). ... For the HFC 134a study, the t(1/2)alpha (alpha elimination half-life) was short for both CFC 12 and HFC 134a (<11 min). The t(1/2)beta (beta elimination half-life) across all exposure concentrations was a mean of 36 and 42 min for CFC 12 and HFC 134a, respectively. ...
PMID:11029265 Emmen HH et al; Regul Toxicol Pharmacol 32 (1): 22-35 (2000)
For more Biological Half-Life (Complete) data for 1,1,1,2-TETRAFLUOROETHANE (8 total), please visit the HSDB record page.