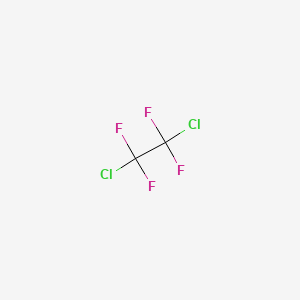

1. 1,2-dichloro-1,1,2,2-tetrafluoroethane
2. 1,2-dichlorotetrafluoroethane
3. Cfc 114
4. Cfc-114
5. Cryofluorane
6. Fluorocarbon 114
7. Freon 114
8. Frigiderm
1. 1,2-dichlorotetrafluoroethane
2. 1,2-dichloro-1,1,2,2-tetrafluoroethane
3. Cryofluorane
4. 76-14-2
5. Cfc-114
6. Refrigerant 114
7. Frigiderm
8. Freon 114
9. Halocarbon 114
10. Propellant 114
11. Fluorocarbon 114
12. Fluorane 114
13. Genetron 114
14. Genetron 316
15. Arcton 33
16. Arcton 114
17. Frigen 114
18. Ledon 114
19. F 114 (halocarbon)
20. R 114 (halocarbon)
21. Ucon 114
22. Ethane, 1,2-dichloro-1,1,2,2-tetrafluoro-
23. Sym-dichlorotetrafluoroethane
24. Cryofluorane [inn]
25. Cryfluorane
26. Fkw 114
27. Ethane, 1,2-dichlorotetrafluoro-
28. Fc 114
29. Tetrafluordichlorethan
30. Cryofluorane (inn)
31. 1,1,2,2-tetrafluoro-1,2-dichloroethane
32. Chlorofluorocarbon 114
33. F 114
34. R 114
35. Nsc-760428
36. 6b5vvt93ar
37. Dichlorotetrafluoroethane [nf]
38. 76-14-21320-37-2(mixedisomers)
39. P-114
40. Dichlorotetrafluoroethane (nf)
41. Cryofluoranum [latin]
42. Caswell No. 326a
43. Criofluorano
44. Cryofluoranum
45. Cryoflurane
46. Cryofluoranum [inn-latin]
47. Criofluorano [inn-spanish]
48. Ccris 8973
49. Hsdb 146
50. Cfc 114
51. Einecs 200-937-7
52. Unii-6b5vvt93ar
53. Epa Pesticide Chemical Code 326200
54. Brn 1740333
55. Cryofluoran
56. Refrigerant R114
57. Isotron 114
58. Cclf2cclf2
59. Dichlortetrafluoroethane
60. Halon 242
61. Flon-114
62. Cryofluorane [mi]
63. S-dichlorotetrafluoroethane
64. Schembl865
65. (cf2cl)2
66. Cryofluorane [mart.]
67. 4-01-00-00137 (beilstein Handbook Reference)
68. Chembl325436
69. Dtxsid8026434
70. Hms3264g16
71. Pharmakon1600-01502370
72. Zinc8214542
73. Dichloro-1,1,2,2-tetrafluoroethane
74. Mfcd00000778
75. Nsc760428
76. Dichlorotetrafluoroethane [ii]
77. 1,2-dichlorotetrafluoro-ethane
78. Akos015915972
79. Ccg-213721
80. Dichlorotetrafluoroethane [hsdb]
81. Nsc 760428
82. Tetrafluordichlorethan [who-dd]
83. Dichlorotetrafluoroethane [vandf]
84. 1,2-dichlorotetrafluoroethane, >=99.5%
85. Db-056030
86. D0417
87. Ft-0606367
88. D03790
89. Ab01563180_01
90. Q420380
91. Sr-01000944235
92. Sr-01000944235-1
93. 1,2-dichlorotetrafluoroethane 100 Microg/ml In Methanol
94. 1,2-dichlorotetrafluoroethane (cfc-114) 2000 Microg/ml In Methanol
| Molecular Weight | 170.92 g/mol |
|---|---|
| Molecular Formula | C2Cl2F4 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 169.9313180 g/mol |
| Monoisotopic Mass | 169.9313180 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 78 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
In various "skin freezes" alone or with other agents by aerosol application. Recommended for spraying of snake & insect bites to retard absorption of venom. /Former/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 165
Human and animal studies indicate rapid excretion of inhaled CFC-114. In a study with radiolabeled CFC-114, 30 minute retention of the dose inhaled in a single breath was 12% versus 23%, 10%, and 20% for comparable doses of trichlorofluoromethane (CFC-11), dichlorodifluoro-methane (CFC-12), and trifluorotrichloroethane (CFC-113), respectively.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's 7th Edition. Dichlorotetrafluoroethane p. 2 CD-ROM Cincinnati, OH 45240-4148 2012.
... Main factor affecting fate of fluorocarbons is body fat, where they are concentrated & slowly released into blood at concn that should not cause any risk of cardiac sensitization. /Fluorocarbons/
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 781
Abosrption of fluorocarbons is much lower after oral ingestion (35-48 times) than after inhalation. ... The lung generally has the highest fluorocarbon concentrations on autopsy. /Fluorocarbons/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 884
Although fluorocarbons cause cardiac sensitization in certain animal species, rapid elimination prevents the development of cardiotoxic concentrations from aerosol bronchodilator use except at exceedingly high doses (12 to 24 doses in 2 minutes). /Fluorocarbons/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 884
For more Absorption, Distribution and Excretion (Complete) data for 1,2-DICHLORO-1,1,2,2-TETRAFLUOROETHANE (7 total), please visit the HSDB record page.