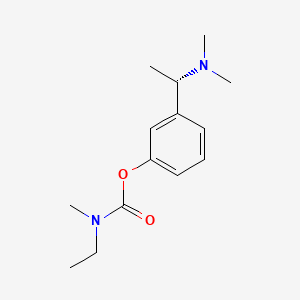

1. (s)-n-ethyl-3-((1-dimethyl-amino)ethyl)-n-methylphenylcarbamate
2. 713, Ena
3. 713, Sdz Ena
4. Ena 713
5. Ena 713, Sdz
6. Ena-713
7. Ena713
8. Exelon
9. Hydrogen Tartrate, Rivastigmine
10. Rivastigmine Hydrogen Tartrate
11. Rivastigminetartrate
12. Sdz Ena 713
13. Tartrate, Rivastigmine Hydrogen
1. 123441-03-2
2. Exelon
3. Ena 713 Free Base
4. Prometax
5. Rivastigmine Teva
6. Nimvastid
7. S-rivastigmine
8. Rivastigmine Hexal
9. (s)-rivastigmine
10. (s)-3-(1-(dimethylamino)ethyl)phenyl Ethyl(methyl)carbamate
11. Rivastigmine Sandoz
12. Sdz-ena-713
13. Sdz-212-713
14. [3-[(1s)-1-(dimethylamino)ethyl]phenyl] N-ethyl-n-methylcarbamate
15. M-((s)-1-(dimethylamino)ethyl)phenyl Ethylmethylcarbamate
16. (s)-3-(1-(dimethylamino)ethyl)phenyl Ethylmethylcarbamate
17. Chembl636
18. 3-[(1s)-1-(dimethylamino)ethyl]phenyl N-ethyl-n-methylcarbamate
19. Pki06m3iw0
20. Chebi:8874
21. Ena-713d
22. Ono-2540
23. Sdz-212713
24. 3-[(1s)-1-(dimethylamino)ethyl]phenyl Ethyl(methyl)carbamate
25. Exelon Patch
26. Ethylmethylcarbamic Acid 3-[(1s)-1-(dimethylamino)ethyl]phenyl Ester
27. Sr-05000001475
28. Unii-pki06m3iw0
29. Sdz 212-713
30. Rivastigmine Transdermal System
31. Prometax (tn)
32. Rivastigmine [usan:inn:ban:jan]
33. Rivastigmine.tartrate
34. Rivastigmine Impurity D
35. 3-((1s)-1-(dimethylamino)ethyl)phenyl Ethylmethylcarbamate
36. Rivastigmine [mi]
37. Rivastigmine [inn]
38. Rivastigmine [jan]
39. Carbamic Acid, Ethylmethyl-, 3-((1s)-1-(dimethylamino)ethyl)phenyl Ester
40. Rivastigmine [usan]
41. Schembl2764
42. Rivastigmine [vandf]
43. Rivastigmine [mart.]
44. Ethylmethylcarbamic Acid 3-[1-(dimethylamino)ethyl]phenyl Ester
45. Mls003876822
46. Bidd:gt0316
47. Rivastigmine [usp-rs]
48. Rivastigmine [who-dd]
49. Rivastigmine (jan/usp/inn)
50. Gtpl6602
51. Zinc4413
52. Rivastigmine [ema Epar]
53. Rivastigmine 1 A Pharma
54. Dtxsid7023564
55. Bdbm10620
56. Bdbm11682
57. Amy3808
58. Hms2089h18
59. Hms3715p15
60. Hms3885b18
61. Rivastigmine [orange Book]
62. Rivastigmine [ep Monograph]
63. Rivastigmine [usp Monograph]
64. Mfcd00871496
65. Akos015896334
66. Carbamic Acid, Ethylmethyl-, 3-(1-(dimethylamino)ethyl)phenyl Ester, (s)-
67. Ac-8250
68. Ccg-221197
69. Cs-0946
70. Db00989
71. Rivastigmine 3m Health Care Ltd
72. Ncgc00167531-03
73. Ncgc00167531-17
74. As-73448
75. Hy-17368
76. Smr002203623
77. R0250
78. S4687
79. (r)-rivastigmine (rivastigmine Ep Impurity D)
80. D03822
81. D82061
82. F10108
83. Ab01275472-01
84. Ab01275472_02
85. Ab01275472_03
86. 101r548
87. Q411887
88. Sr-05000001475-1
89. Sr-05000001475-2
90. W-200966
91. (s)-3-[1-(dimethylamino)ethyl]phenyl N-ethyl-n-methylcarbamate
92. [3-[(1s)-1-dimethylaminoethyl]phenyl] N-ethyl-n-methylcarbamate
93. 3-((1s)-1-(dimethylamino)ethyl)phenyl N-ethyl N-methyl Carbamate
94. Ethyl-methyl-carbamic Acid 3-(1-dimethylamino-ethyl)-phenyl Ester
95. Ethyl-methyl-carbamic Acid 3-((r)-1-dimethylamino-ethyl)-phenyl Ester
96. Ethyl-methyl-carbamic Acid 3-((s)-1-dimethylamino-ethyl)-phenyl Ester
97. Carbamic Acid, N-ethyl-n-methyl-, 3-[(1s)-1-(dimethylamino)ethyl]phenyl Ester
98. 2,3-dihydroxybutanedioic Acid; 3-[(1s)-1-(dimethylamino)ethyl]phenyl N-ethyl-n-methylcarbamate
| Molecular Weight | 250.34 g/mol |
|---|---|
| Molecular Formula | C14H22N2O2 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 250.168127949 g/mol |
| Monoisotopic Mass | 250.168127949 g/mol |
| Topological Polar Surface Area | 32.8 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 269 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 3 | |
|---|---|
| Drug Name | EXELON |
| Active Ingredient | RIVASTIGMINE TARTRATE |
| Company | NOVARTIS (Application Number: N020823) |
| 2 of 3 | |
|---|---|
| Drug Name | EXELON |
| Active Ingredient | RIVASTIGMINE |
| Company | NOVARTIS (Application Number: N022083. Patents: 6316023, 6335031) |
| 3 of 3 | |
|---|---|
| Drug Name | RIVASTIGMINE |
| Active Ingredient | RIVASTIGMINE |
| Company | ALVOGEN MALTA (Application Number: A204403) |
For the treatment of mild to moderate dementia associated with Parkinson's disease or of the Alzheimer's type.
FDA Label
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Treatment of dementia
Treatment of dementia
Rivastigmine is a parasympathomimetic and a reversible cholinesterase inhibitor. An early pathophysiological feature of Alzheimer's disease that is associated with memory loss and cognitive deficits is a deficiency of acetylcholine as a result of selective loss of cholinergic neurons in the cerebral cortex, nucleus basalis, and hippocampus. Tacrine is postulated to exert its therapeutic effect by enhancing cholinergic function. While the precise mechanism of rivastigmine's action is unknown, it is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. If this proposed mechanism is correct, rivastigmine's effect may lessen as the disease progresses and fewer cholinergic neurons remain functionally intact.
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
N06DA03
N06DA03
N06DA03
N06DA03
N06DA03
N06DA03
N06DA03
N06DA03
N06DA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06D - Anti-dementia drugs
N06DA - Anticholinesterases
N06DA03 - Rivastigmine
Route of Elimination
Rivastigmine is extensively metabolized primarily via cholinesterase-mediated hydrolysis to the decarbamylated metabolite NAP226-90. Renal excretion of the metabolites is the major route of elimination. Less than 1% of the administered dose is excreted in the feces.
Volume of Distribution
1.8 to 2.7 L/kg
Clearance
renal cl=2.1-2.8 L/hr
Rivastigmine is rapidly metabolized by cholinesterase-mediated hydrolysis.
1.5 hours
Rivastigmine is a carbamate derivative that is structurally related to physostigmine, but not to donepezil and tacrine. The precise mechanism of rivastigmine has not been fully determined, but it is suggested that rivastigmine binds reversibly with and inactivates chlolinesterase (eg. acetylcholinesterase, butyrylcholinesterase), preventing the hydrolysis of acetycholine, and thus leading to an increased concentration of acetylcholine at cholinergic synapses. The anticholinesterase activity of rivastigmine is relatively specific for brain acetylcholinesterase and butyrylcholinesterase compared with those in peripheral tissues.