
1. Galantamin
2. Galanthamine
3. Galanthamine Hydrobromide
4. Lycoremine
5. Nivalin
6. Nivaline
7. Razadyne
8. Reminyl
1. Galanthamine
2. 357-70-0
3. (-)-galanthamine
4. Lycoremin
5. Lycoremine
6. Jilkon
7. (-)-galantamine
8. Razadyne
9. (+/-)-galantamine
10. Razadyne Er
11. Bodamine
12. (+/-)-galanthamine
13. Galantamina
14. Reminyl (tn)
15. Galantamine, (+/-)-
16. Galanthamine, (+/-)-
17. Chembl659
18. 0d3q044kca
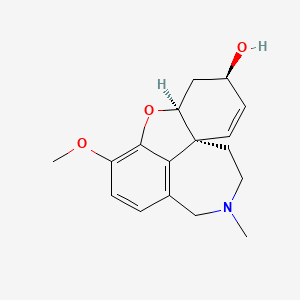
19. (4as,6r,8as)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol
20. Galanthaminum
21. Chebi:42944
22. 1t835z585r
23. Nsc-100058
24. 23173-12-8
25. Galanthamine Hbr
26. Ncgc00017256-04
27. Galantaminum
28. Galantaminum [inn-latin]
29. Gnt
30. Galantamina [inn-spanish]
31. (1s,12s,14r)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.0^{1,12}.0^{6,17}]heptadeca-6,8,10(17),15-tetraen-14-ol
32. (1s,12s,14r)-9-methoxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-14-ol
33. (4as,6r,8as)-3-methoxy-11-methyl-5,6,9,10,11,12-hexahydro-4ah-benzo[2,3]benzofuro[4,3-cd]azepin-6-ol
34. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, (4ar,6s,8ar)-rel-
35. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, (4as,6r,8as)-
36. 6h-benzofuro[3a,3,2-ef][2]benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, (4as,6r,8as)-
37. Nsc 100058
38. Brn 0093736
39. Hsdb 7361
40. Galantamine (usan/inn)
41. Unii-0d3q044kca
42. Galantamine [usan:inn:ban]
43. Sr-05000001783
44. (-)galanthamine
45. 1qti
46. Galanthamine, 12
47. Spectrum_001271
48. 1dx6
49. Galantamine [mi]
50. Prestwick0_000588
51. Prestwick1_000588
52. Prestwick2_000588
53. Prestwick3_000588
54. Spectrum3_001738
55. Spectrum4_000839
56. Spectrum5_001673
57. Galantamine [inn]
58. Galantamine [hsdb]
59. Galantamine [usan]
60. Probes1_000055
61. Probes2_000395
62. Galantamine [vandf]
63. Schembl2577
64. Dsstox_cid_25606
65. Dsstox_rid_80999
66. Dsstox_gsid_45606
67. Bspbio_000436
68. Bspbio_003416
69. Galantamine [who-dd]
70. Kbiogr_001417
71. Kbioss_001751
72. 4-27-00-02184 (beilstein Handbook Reference)
73. Bidd:gt0517
74. Divk1c_000590
75. Spbio_002655
76. Bpbio1_000480
77. Gtpl6693
78. Schembl3293474
79. Dtxsid2045606
80. Unii-1t835z585r
81. Asutzqlvashgkv-jdfrzjqesa-
82. Bdbm10404
83. Kbio1_000590
84. Kbio2_001751
85. Kbio2_004319
86. Kbio2_006887
87. Kbio3_002636
88. Ninds_000590
89. Hms2089h03
90. Hms3885c10
91. Pharmakon1600-01501202
92. Zinc491073
93. Tox21_110807
94. Mfcd00867189
95. Nsc759861
96. S3866
97. Akos015965330
98. Am62710
99. Ccg-212961
100. Cs-1217
101. Db00674
102. Nsc-759861
103. Sdccgmls-0066737.p001
104. Idi1_000590
105. Smp1_000131
106. Ncgc00017256-05
107. Ncgc00017256-11
108. Ncgc00017256-17
109. Ncgc00024731-02
110. (4as,6r,8as)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6h-benzofurol[3a,3,2,-ef][2]benzazepin-6-ol
111. Ac-20240
112. Ac-34328
113. As-56354
114. Cas-357-70-0
115. Hy-76299
116. Sbi-0051689.p002
117. C08526
118. D04292
119. Ab00053614-09
120. Ab00053614_10
121. 357g700
122. Q412690
123. Sr-05000001783-4
124. Brd-k49481516-004-03-5
125. Brd-k49481516-004-04-3
126. Brd-k49481516-004-09-2
127. (4as,6r,8as)-3-methoxy-11-methyl-4a,5,9,10,11,12-hexahydro-6h-benzo[2,3]benzofuro[4,3-cd]azepin-6-ol
128. 1008759-59-8
129. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, (4a.alpha.,6.beta.,8ar*)-
130. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, (4aalpha,6beta,8ar*)-
131. 6h-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol, 4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-, (4as,6r,8as)
| Molecular Weight | 287.35 g/mol |
|---|---|
| Molecular Formula | C17H21NO3 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 287.15214353 g/mol |
| Monoisotopic Mass | 287.15214353 g/mol |
| Topological Polar Surface Area | 41.9 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | RAZADYNE ER |
| Active Ingredient | GALANTAMINE HYDROBROMIDE |
| Company | JANSSEN PHARMS (Application Number: N021615. Patent: 7160559) |
| 2 of 2 | |
|---|---|
| Drug Name | RAZADYNE |
| Active Ingredient | GALANTAMINE HYDROBROMIDE |
| Company | JANSSEN PHARMS (Application Number: N021169) |
Cholinesterase inhibitor
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 771
Galanatamine is indicated for the treatment of mild to moderate dementia of the Alzheimer's type. / Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506
In two randomized placebo controlled trials of 2 years duration in subjects with mild cognitive impairment (MCI), a total of 13 subjects on razadyne (n=1026) and 1 subject on placebo (n=1022) died. The deaths were due to various causes which could be expected in an elderly population; about half of the razadyne deaths appeared to result from various vascular causes (myocardial infarction, stroke, and sudden death). Although the difference in mortality between razadyne and placebo-treated groups in these two studies was significant, the results are highly discrepant with other studies of razadyne. Specifically, in these two MCI studies, the mortality rate in the placebo-treated subjects was markedly lower than the rate in placebo-treated patients in trials of razadyne in Alzheimer's disease or other dementias (0.7 per 1000 person years compared to 22-61 per 1000 person years, respectively). Although the mortality rate in the razadyne-treated MCI subjects was also lower than that observed in razadyne -treated patients in Alzheimer's disease and other dementia trials (10.2 per 1000 person years compared to 23-31 per 1000 person years, respectively), the relative difference was much less. When the Alzheimer's disease and other dementia studies were pooled (n=6000), the mortality rate in the placebo group numerically exceeded that in the razadyne group. Furthermore, in the MCI studies, no subjects in the placebo group died after 6 months, a highly unexpected finding in this population. Individuals with mild cognitive impairment demonstrate isolated memory impairment greater than expected for their age and education, but do not meet current diagnostic criteria for Alzheimer's disease.
Ortho-McNeil Neurologics Inc.; Full US Prescribing Information for Razadyne (Revised June 2005) Available from, as of October 26, 2005: https://www.razadyne.com/active/janus/en_US/assets/common/company/pi/razadyne.pdf
FDA Pregnancy Risk Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506
Potential for increased risk of seizures secondary to cholinergic activity (seizures also may be a manifestation of Alzheimer's disease).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 1213
Adverse effects reported in 5% or more of patients receiving galantamine hydrobromide and with an incidence of at least twice that of placebo include nausea, vomiting, diarrhea, anorexia, weight decrease. Most of these adverse effects occurred during the upward titration of dosages. Administration of galantamine with food, use of antiemetic agents, and ensuring adequate fluid intake may reduce the impact of these adverse events.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 1214
For more Drug Warnings (Complete) data for GALANTAMINE (14 total), please visit the HSDB record page.
Galantamine is indicated for the treatment of mild to moderate dementia of the Alzheimers type.
FDA Label
Galantamine is a competitive and reversible inhibitor of acetylcholinesterase that works to increase acetylcholine levels. Galantamine acts both centrally and peripherally to inhibit both muscle and brain acetylcholinesterase, thereby increasing cholinergic tone. Galantamine is also a positive allosteric modulator of neuronal nicotinic acetylcholine receptors. As dementia is a progressive neurodegenerative disease, galatamine has a negligible effect in altering the course of the underlying process of dementia and may exert its therapeutic effectiveness for a short period of time. However, galantamine promoted improvements in cognition, global function, activities of daily living, and behavioural symptoms in clinical studies of Alzheimers disease. Galantamine exhibited therapeutic efficacy in studies of vascular dementia and Alzheimers disease with cerebrovascular disease. In one study, galantamine reversed scopolamine-induced acute anticholinergic syndrome that was characterized by drowsiness, disorientation, and delirium.
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Nootropic Agents
Drugs used to specifically facilitate learning or memory, particularly to prevent the cognitive deficits associated with dementias. These drugs act by a variety of mechanisms. (See all compounds classified as Nootropic Agents.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)
N06DA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06D - Anti-dementia drugs
N06DA - Anticholinesterases
N06DA04 - Galantamine
Absorption
Over a dose range of 8-32 mg/day, galantamine exhibits a dose-linear pharmacokinetic profile. The oral bioavailability of galantamine ranges from 90-100%. Following oral administration, the Tmax is about 1 hour. Following 10 hours of administration, the mean galantamine plasma concentrations were 8297 g/L for the 24 mg/day dose and 114126 g/L for the 32 mg/day dose.
Route of Elimination
Renal clearance accounts for about 2025% of total plasma clearance of the drug in healthy individuals: the elimination of galantamine has been shown to be decreased in subjects with renal impairment. Following oral or intravenous administration, approximately 20% of the dose is excreted as unchanged in the urine within 24 h. In a radiolabelled drug study, about 95% and 5% of the total radioactivity was recovered in the urine and feces, respectively. Of the dose recovered in the urine, about 32% was in the unchanged parent compound, and 12% was in the glucuronide form.
Volume of Distribution
The mean volume of distribution is 175 L. About 52.7% of galantamine is distributed to blood cells, the blood to plasma concentration ratio of galantamine is 1.2. Galantamine penetrates the bloodbrain barrier.
Clearance
The renal clearance is 65 mL/min and the total plasma clearance is about 300 mL/min.
Protein binding: Low (18%)
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506
Mean volume of distribution is 175 L.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506
The maximum inhibition of acetylcholinesterase activity of about 40% was achieved about one hour after a single oral dose of 8 mg galantamine in healthy male subjects.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1737
Galantamine is rapidly and completely absorbed. The absolute oral bioavailability is about 90%. Galantamine shows linear pharmacokinetics with doses ranging from 8 to 32 mg/day.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506
For more Absorption, Distribution and Excretion (Complete) data for GALANTAMINE (6 total), please visit the HSDB record page.
_In vitro_ study findings suggest that about 75% of the drug is metabolized by CYP2D6 and CYP3A4. CYP2D6 promotes O-demethylation of the drug to form O-desmethyl-galantamine and the CYP3A4-mediated pathway forms the galantamine-N-oxide. Important metabolic pathways also include N-demethylation, epimerization, and sulfate conjugation. Other metabolites include norgalantamine, O-desmethyl-galantamine, O-desmethyl-norgalantamine, epigalantamine and galantaminone, which do not retain clinically significant pharmacology activities. Galantamine can also undergo glucuronidation: in one oral radiolabeled drug study in poor and extensive CYP2D6 metabolizers, about 14-24% of the total radioactivity was identified as galantamine glucuronide 8 hours post-dose. O-demethylation by CYP2D6 becomes prominent in patients with who are extensive metabolizers of CYP2D6, but unchanged galatamine (39-77%) and its glucuronide metabolite (14-24%) predominated in the plasma of both poor and extensive metabolizers of CYP2D6 in a radiolabelled drug study. The total plasma clearance, or nonrenal clearnace, accounts for 2025% of drug elimination.
In studies of oral 3(H)-galantamine, unchanged galantamine and its glucuronide, accounted for most plasma radioactivity in poor and extensive CYP2D6 metabolizers. Up to 8 hours post-dose, unchanged galantamine accounted for 39-77% of the total radioactivity in the plasma, and galantamine glucuronide for 14-24%. By 7 days, 93- 99% of the radioactivity had been recovered, with about 95% in urine and about 5% in the feces. Total urinary recovery of unchanged galantamine accounted for, on average, 32% of the dose and that of galantamine glucuronide for another 12% on average.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1727
Galantamine is metabolized by hepatic cytochrome P450 enzymes, glucuronidated, and excreted unchanged in the urine. In vitro studies indicate that cytochrome CYP2D6 and CYP3A4 were the major cytochrome P450 isoenzymes involved in the metabolism of galantamine, and inhibitors of both pathways increase oral bioavailability of galantamine modestly. O-demethylation, mediated by CYP2D6 was greater in extensive metabolizers of CYP2D6 than in poor metabolizers. In plasma from both poor and extensive metabolizers, however, unchanged galantamine and its glucuronide accounted for most of the sample radioactivity.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1737
Galantamine is metabolized by hepatic cytochrome p450 enzymes.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506
Galantamine has known human metabolites that include Galantamine N-oxide, N-desmethylgalantamine, O-Desmethylgalantamine, and [(1S,12S,14R)-14-hydroxy-4-methyl-11-oxa-4-azatetracyclo[8.6.1.01,12.06,17]heptadeca-6(17),7,9,15-tetraen-9-yl] hydrogen sulfate.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Galantamine has a terminal half-life of about 7 hours.
Elimination half-life: 7 hours
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506
Alzheimers disease is characterized by progressive, irreversible degeneration of acetylcholine-producing neurons, cognitive impairment, and the accumulation of neurofibrillary tangles and amyloid plaques. The cholinergic system plays a critical role in memory, alongside other important neural functions such as attention, learning, stress response, wakefulness and sleep, and sensory information. Studies show that acetylcholine (ACh) is involved in the modulation of acquisition, encoding, consolidation, reconsolidation, extinction, and retrieval of memory. The gradual loss of cholinergic neurons in Alzheimers disease (AD) may, therefore, contribute to the memory loss exhibited by AD patients. Acetylcholinesterase is secreted by cholinergic neurons to rapidly hydrolyze ACh at the synaptic cleft to release acetate and choline. Choline is later recycled back into the presynaptic cholinergic neuron via reuptake by the high-affinity choline transporter. There is some evidence demonstrating the potential involvement of the acetylcholinesterase enzyme in the formation of amyloid fibrils. Galantamine competitively and reversibly inhibits the anticholinesterase enzyme in the CNS (namely in the frontal cortex and hippocampal regions) by binding to the choline-binding site and acyl-binding pocket of the enzyme active site. By blocking the breakdown of ACh, galantamine enhances ACh levels in the synaptic cleft. Nicotinic acetylcholine receptors (nAChR) in the CNS are mostly expressed at the presynaptic neuronal membrane to control the release of multiple neurotransmitters, such as ACh, glutamate, GABA, dopamine, serotonin, norepinephrine. Agonists of nAChRs improve performance in cognitive tasks, while antagonists of nAChR impair cognitive processes. Some studies show a decrease in the expression and activity of nAChRs in patients with AD, which may explain the reduction in central cholinergic neurotransmission in these patients. Galantamine binds to nAChRs at the allosteric site, leading to a conformational change of the receptor, increased ACh release, and increased activity of neighbouring glutaminergic and serotoninergic neurons. The modulation of nAChRs facilitates both excitatory and inhibitory cholinergic transmissions in brain tissues and increases receptor sensitivity. The modulated release of other neurotransmitters by galantamine may also contribute to the upregulation of nAChRs and amelioration of behavioural symptoms in AD.
Galantamine, a tertiary alkaloid, is a competitive and reversible inhibitor of acetylcholinesterase. While the precise mechanism of galantamine's action is unknown, it is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. If this mechanism is correct, galantamine's effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that galantamine alters the course of the underlying dementing process.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1737
The cause of cognitive impairment in Alzheimer's Disease is not fully understood, it has been shown that acetylcholine producing neurons degenerate. The cholinergic loss has been correlated with cognitive impairment and a density of amyloid plaques. Galantamine is a tertiary alkaloid and it competes with and is a reversible inhibitor of acetylcholinesterase. The exact mechanism of galantamine is not known, but it is believed to enhance cholinergic function.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 1506