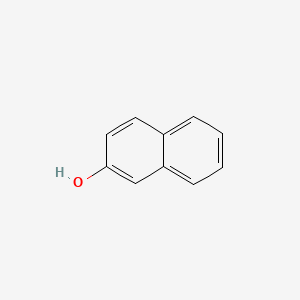

1. 2-hydroxynaphthalene
2. 2-naphthol, (1+)
3. 2-naphthol, 1,4,5,8-(14)c4-labeled
4. 2-naphthol, 7-(14)c-labeled
5. 2-naphthol, 8-(14)c-labeled
6. 2-naphthol, Bismuth Salt
7. 2-naphthol, Magnesium Salt
8. 2-naphthol, Potassium Salt
9. 2-naphthol, Sodium Salt
10. 2-naphthol, Titanium(4+) Salt
11. Beta-naphthol
1. Naphthalen-2-ol
2. 135-19-3
3. Beta-naphthol
4. 2-naphthalenol
5. Betanaphthol
6. 2-hydroxynaphthalene
7. Isonaphthol
8. Developer Bn
9. Naphthol B
10. 2-naphtol
11. Azogen Developer A
12. Beta-naphtol
13. Beta-napthol
14. Developer A
15. Developer Ams
16. Beta-hydroxynaphthalene
17. 2-naftolo
18. 2-naftol
19. Developer Sodium
20. C.i. Developer 5
21. .beta.-naphthol
22. Beta-naphthyl Alcohol
23. Beta-monoxynaphthalene
24. Beta-naftol
25. Beta-naftolo
26. Beta-naphthyl Hydroxide
27. Naphthol, Beta
28. C.i. Azoic Coupling Component 1
29. .beta.-hydroxynaphthalene
30. Antioxygene Bn
31. .beta.-naftolo
32. .beta.-naphtol
33. .beta.-napthol
34. .beta.-naftol
35. Nsc 2044
36. C.i. 37500
37. Mfcd00004067
38. .beta.-naphthyl Alcohol
39. .beta.-monoxynaphthalene
40. .beta.-naphthyl Hydroxide
41. 2-naphthalenol, Homopolymer
42. P2z71cik5h
43. Chembl14126
44. Chebi:10432
45. Naphthalen-2-ol (beta-naphthol)
46. Nsc-2044
47. Dsstox_cid_7061
48. Dsstox_rid_78296
49. Dsstox_gsid_27061
50. 26716-78-9
51. 2-naftol [dutch]
52. Beta-naftol [dutch]
53. 2-naphtol [french]
54. Caswell No. 590
55. 2-naftolo [italian]
56. Beta-naphtol [german]
57. Beta-naftolo [italian]
58. 2-napththol
59. Cas-135-19-3
60. Betanaphthol [nf]
61. Hsdb 6812
62. Einecs 205-182-7
63. Unii-p2z71cik5h
64. Epa Pesticide Chemical Code 010301
65. Hydronaphthol
66. Ai3-00081
67. 2-napthol
68. Napthalen-2-ol
69. 2-naphthylalcohol
70. Naphth-2-ol
71. Naphthol As-ptr
72. Naphthol, .beta.
73. 03v
74. Einecs 215-322-9
75. 2-hydroxy Naphthalene
76. 2-naphthol, Reagent
77. 2-naphthol, Purified
78. Brn 1817321
79. 2-naphthol, 98%
80. 2-naphthol, 99%
81. Naphth-2-ol, 10
82. Beta.-hydroxynaphthalene
83. Microcidin (salt/mix)
84. Betanaphthol [ii]
85. 2-naphthol [mi]
86. 2-naphthol, 98.5%
87. Betanaphthol [hsdb]
88. Ec 205-182-7
89. 2-naphthol [inci]
90. Schembl28781
91. Wln: L66j Cq
92. Betanaphthol [mart.]
93. Betanaphthol [who-dd]
94. 2-naphthol(flakes Or Chunks)
95. Sgcut00131
96. 2-naphthol, Lr, >=98%
97. Dtxsid5027061
98. Nsc2044
99. 4b32
100. Sodium 2-naphthoxide (salt/mix)
101. Hms3264n15
102. Pharmakon1600-01504501
103. Zinc967928
104. Hy-y0110
105. To_000010
106. Tox21_201884
107. Tox21_303038
108. Bdbm50159250
109. Nsc758883
110. S6035
111. Stl281866
112. Akos000119842
113. Am86551
114. Ccg-213932
115. Hr-0304
116. Nsc-758883
117. 2-naphthol 1000 Microg/ml In Methanol
118. 2-naphthol, Purum, >=98.0% (gc)
119. Ncgc00249132-01
120. Ncgc00257077-01
121. Ncgc00259433-01
122. Ac-10464
123. 2-naphthol, Bioxtra, >=99.0% (gc)
124. Sbi-0207084.p001
125. 2-naphthol, Saj First Grade, >=98.0%
126. Tolnaftate Impurity A [ep Impurity]
127. Cs-0008403
128. Ft-0613121
129. N0027
130. 2-naphthol, Fluorescence Indicator, >=99.0%
131. D86186
132. Ab01314260_03
133. 4-06-00-04208 (beilstein Handbook Reference)
134. A806896
135. Ab-131/40299865
136. Q949232
137. Sr-01000872753
138. Q-200736
139. Sr-01000872753-1
140. Brd-k21164796-001-01-0
141. Z57127515
142. F0001-0455
| Molecular Weight | 144.17 g/mol |
|---|---|
| Molecular Formula | C10H8O |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 144.057514874 g/mol |
| Monoisotopic Mass | 144.057514874 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 133 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
2-Naphthol ... has had medical uses as a counterirritant in alopecia, also as an anthelmintic, and as an antiseptic in treatment of scabies.
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 655
2-Naphthol-containing pastes should be applied only for short periods of time and to a limited area not exceeding 150 square cm.
Marzulli, F.N., H.I. Maibach. Dermatotoxicology 4th ed. New York, NY: Hemisphere Publishing Corp., 1991., p. 864
Between 5 and 10% of a cutaneous dose /of 2-naphthol/ has been recovered from the urine ... .
Marzulli, F.N., H.I. Maibach. Dermatotoxicology 4th ed. New York, NY: Hemisphere Publishing Corp., 1991., p. 864
Several pathways of drug metabolizing enzyme activity were measured in hepatic fractions of cattle, sheep, goats, chickens, turkeys, ducks, rabbits and rats. The pathways examined included the O-demethylation of p-nitrophenol, microsomal ester hydrolysis of procaine and glucuronidation of p-nitrophenol, and the cytosolic acetylation of sulfamethazine and sulfation of 2-naphthol. For most enzymatic pathways measured, goats were more similar to sheep (wether) than to cattle (steers). The exception was UDP-glucuronyltransferase activity, which was significantly higher for the goat than for any other species studied. Within the avian subset, the chicken and turkey were usually the most similar species. The activities of arylsulfotransferase isozymes III and IV were particularly low for the duck compared to the chicken and turkey. N-acetyltransferase activity was very high for rabbits and very low for sheep and goats.
PMID:2905957 Short CR et al; Comp Biochem Physiol C 91 (2): 419-24 (1988)
Several pathways of drug metabolizing enzymic activity were measured in hepatic fractions of the channel catfish and rat using model substrates. The pathways examined included the O-demethylation of p-nitroanisole, microsomal ester hydrolysis of procaine and glucuronidation of p-nitrophenol and the cytosolic acetylation of sulfamethazine and sulfation of 2-naphthol. Catfish liver preparations were incubated at both 25 C and 37 C. The oxidative metabolism of p-nitrophenol was only 1/8 of that of the rat at 37 C and 1/12 that of the rat at 25 C. Procaine ester hydrolysis was negligible in catfish microsomal preparations. At 37 C, p-nitrophenol glucuronidation was equivalent in catfish and rat microsomes. Catfish cytosolic preparations exhibited N-acetyltransferase and arylsulfotransferase nearly comparable to those of the rat. Rates of glucuronidation and sulfation were higher at 37 C than at 25 C in hepatic fractions of the catfish.
PMID:2898990 Short CR et al; Comp Biochem Physiol C 89 (2): 153-7 (1988)
To characterize the substrate specificities of various isozymes of carboxylesterases, a series of carbonates, thiocarbonates, carbamates, and carboxylic acid esters containing alpha- or beta-naphthol or p-nitrophenol as leaving groups were tested as substrates of human, rat and mouse liver microsomal esterases; hydrolases A and B from rat liver microsomes were also tested. The carbonates, thiocarbonates, and carboxylic esters of alpha-naphthol were cleaved more rapidly than the corresponding beta-naphthol isomers by the mammalian liver esterases. The majority of the substrates was consistently hydrolyzed at higher rates by hydrolase B compared with hydrolase A. Compared with the corresponding carboxylates, the carbonate moiety of alpha- and beta-naphthol and p-nitrophenol lowered the specific activities of the enzymes by about 5 fold but improved stability under basic conditions. Human and mouse liver microsomal esterase activities were 5 orders of magnitude lower than the esterase activities of hydrolase B. The functional group and lipophilicity of the substrate structure influenced the activity of mammalian esterases.
PMID:8321828 Huang TL et al; Pharm Res 10: 639-48 (1993)
The inhibition of hydroxysteroid-sulfotransferase (ST) activity in the rat liver by alkylamines was investigated. Liver homogenates were prepared from Wistar rats, and cytosolic fractions were obtained. ST activities towards dehydroepiandrosterone (DHEA), androsterone (AS), and 2-naphthol (2NA) were assayed. Cytosolic fractions were fractionated by column chromatography. Triethylamine, which was used as an elution solvent for column chromatography to purify chemically synthesized 3-phosphoadenosine-5-phosphosulfate (PAPS) inhibited androgen sulfation with AS and DHEA, but did not affect ST activities with cortisol and 2-NA. The sulfate donor ability of various PAPS preparations were compared. Fourteen primary, secondary, and tertiary amines were examined for inhibitory actions on ST activities towards DHEA, cortisol, and 2-NA. A secondary amine, di-n-butylamine, and three tertiary amines, triethylamine, tri-n-propylamine and tri-n-butylamine, inhibited DHEA ST activity by 40 to 60%, irrespective of sex. However, 2-NA and cortisol ST activities were not affected to any significant extent. Lineweaver Burk plots with partially purified hydroxysteroid ST indicated that the inhibition by triethylamine fitted a noncompetitive inhibition. The /results/ conclude that glucocorticoid ST appears to be distinct from the hydroxysteroid ST, and that this has implications for the inhibition of human liver ST activities by synthetic steroids and tertiary amines given as drugs.
Matsui M et al; Biochem Pharmacology 46 (3): 465-70 (1993)
For more Metabolism/Metabolites (Complete) data for 2-NAPHTHOL (8 total), please visit the HSDB record page.
2-Naphthol is a known human metabolite of naphthalene.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560