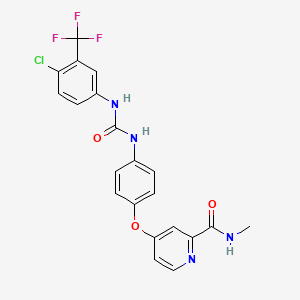

1. 4-(4-(3-(4-chloro-3-trifluoromethylphenyl)ureido)phenoxy)pyridine-2-carboxylic Acid Methyamide-4-methylbenzenesulfonate
2. Bay 43 9006
3. Bay 43-9006
4. Bay 439006
5. Bay 545 9085
6. Bay 545-9085
7. Bay 5459085
8. Bay 673472
9. Bay-545-9085
10. Bay-673472
11. Bay5459085
12. Nexavar
13. Sorafenib N Oxide
14. Sorafenib N-oxide
15. Sorafenib Tosylate
1. 284461-73-0
2. Nexavar
3. Bay 43-9006
4. 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-n-methylpicolinamide
5. 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-n-methylpyridine-2-carboxamide
6. Sorafenibum
7. 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-n-methylpyridine-2-carboxamide
8. Sorafenib Free Base
9. 100012-18-8
10. N-(4-chloro-3-(trifluoromethyl)phenyl)-n'-(4-(2-(n-methylcarbamoyl)-4-pyridyloxy)phenyl)urea
11. 4-(4-((((4-chloro-3-(trifluoromethyl)phenyl)amino)carbonyl)amino)phenoxy)-n-methyl-2-pyridinecarboxamide
12. 284461-73-0 (free Base)
13. Mfcd06411450
14. Bay-43-9006
15. 9zoq3tzi87
16. Chembl1336
17. 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-n-methyl-pyridine-2-carboxamide
18. 4-{4-[({[4-chloro-3-(trifluoromethyl)phenyl]amino}carbonyl)amino]phenoxy}-n-methylpyridine-2-carboxamide
19. Dtxsid7041128
20. Chebi:50924
21. Sorafenib [inn]
22. 2-pyridinecarboxamide, 4-(4-((((4-chloro-3-(trifluoromethyl)phenyl)amino)carbonyl)amino)phenoxy)-n-methyl-
23. 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-n-methyl-2-pyridinecarboxamide
24. Sorafenib (nexavar)
25. 4-(4-{3-(4-chloro-3-(trifluoromethyl)phenyl)ureido}phenoxy)-n(sup 2)-methylpyridine-2-carboxamide
26. Donafenib (sorafenib D3)
27. 2-pyridinecarboxamide, 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-n-methyl-
28. Bax
29. Manganese(4+), Chloro[[4,4',4'',4'''-(21h,23h-porphine-5,10,15,20-tetrayl-kappan21,kappan22,kappan23,kappan24)tetrakis[1-methylpyridiniumato]](2-)]-, Chloride (1:4), (sp-5-12)-
30. 1210608-86-8
31. Nsc-724772
32. Ncgc00167488-01
33. Unii-9zoq3tzi87
34. Sorafenib [usan:inn:ban]
35. Sr-00000000529
36. Hsdb 8173
37. 1uwh
38. 3gcs
39. 3heg
40. 4asd
41. Hit Compound, 8
42. Sorafenib, 4
43. 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-n(sup 2)-methylpyridine-2-carboxamide
44. Bay 439006
45. Bay43-9006
46. Kinome_766
47. Sorafenib Base
48. Sorafenib [mi]
49. Sorafenib (usan/inn)
50. Sorafenib-[13c,d3]
51. Sorafenib [usan]
52. Nexavar (tn) (bayer)
53. Sorafenib [vandf]
54. Sorafenib [mart.]
55. Ec 608-209-4
56. Schembl8218
57. Sorafenib [who-dd]
58. Sorafenib [ema Epar]
59. Bay 43-9006; Sorafenib
60. Cid_216239
61. Gtpl5711
62. Bdbm16673
63. Bcpp000064
64. Hms2043a18
65. Hms3244a15
66. Hms3244a16
67. Hms3244b15
68. Hms3656n20
69. K00597a
70. Act06732
71. Bcp01767
72. Bcp34023
73. Ex-a2894
74. Zinc1493878
75. Bay439006
76. Nsc747971
77. Nsc800934
78. S7397
79. Stk627350
80. Akos005560229
81. Ac-1674
82. Bay-439006
83. Ccg-269400
84. Cs-1590
85. Db00398
86. Nsc-747971
87. Nsc-800934
88. Pb14443
89. Sb19942
90. Sf-0529
91. Bay-43-0006
92. Sorafenib Free Base (bay-43-9006)
93. Ncgc00167488-02
94. Ncgc00167488-03
95. Ncgc00167488-04
96. Ncgc00167488-05
97. Ncgc00167488-07
98. Ncgc00167488-14
99. 4(4-{3-[4-chloro-3-(trifluoromethyl)phenyl]ureido}phenoxy)-n(sup 2)-methylpyridine-2-carboxamide
100. Bs164413
101. Hy-10201
102. N-(4-chloro-3-(trifluoromethyl)phenyl)-n'-(4-(2-(n-methylcar Bamoyl)-4-pyridyloxy)phenyl)urea
103. Sy009239
104. Am20090614
105. Ft-0650736
106. Ft-0674632
107. Sw202562-4
108. D08524
109. Ab00933189-05
110. Ab00933189-06
111. Ab00933189_08
112. 461s730
113. A819449
114. Q421136
115. Q-201728
116. Sr-00000000529-1
117. Brd-k23984367-001-01-8
118. Z89277543
119. Bay 439006; Bay439006; Bay-439006
120. Sorafenib (d3); Cm-4307; Cm 4307; Cm4307;bay 43-9006 (d3)
121. 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl) Ureido) Phenoxy)-n-methylpicolinamide
122. 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl) Ureido)phenoxy)-n-methylpicolinamide
123. N-(3-trifluoromethyl-4-chlorophenyl)-n'-(4-(2-methylcarbamoyl Pyridin-4-yl)oxyphenyl)urea
124. 4-(4-{3-[4-chloro-3-(trifluoromethyl)phenyl]ureido}phenoxy)- N-methylpyridine-2-carboxamide
125. 4-(4-{3-[4-chloro-3-(trifluoromethyl)phenyl]ureido}phenoxy)-n2-methylpyridine-2-carboxamide
126. 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-n-methyl-picolinamide;tosylic Acid
127. 4-{4-[({[4-chloro-3-(trifluoromethyl)phenyl]amino}carbonyl)-amino]phenoxy}-n-methylpyridine-2-carboxamide
128. 4-{4-[({[4-chloro-3-(trifluoromethyl)phenyl]amino}carbonyl)amino]phenoxy}-n-methyl-pyridine-2-carboxamide
129. N-(4-chloro-3-(trifluoromethyl)phenyl)-n'-(4-(2-(n-methylcarbamoyl)-4-pyridyloxy)phenyl) Urea
| Molecular Weight | 464.8 g/mol |
|---|---|
| Molecular Formula | C21H16ClF3N4O3 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 464.0863026 g/mol |
| Monoisotopic Mass | 464.0863026 g/mol |
| Topological Polar Surface Area | 92.4 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 646 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | NEXAVAR |
| Active Ingredient | SORAFENIB TOSYLATE |
| Company | BAYER HLTHCARE (Application Number: N021923. Patents: 7235576, 7351834, 7897623, 8124630, 8618141, 8841330, 8877933, 9737488) |
Antineoplastic Agents; Protein Kinase Inhibitors
National Library of Medicine's Medical Subject Headings. Sorafenib. Online file (MeSH, 2014). Available from, as of January 30, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Nexavar is indicated for the treatment of patients with unresectable hepatocellular carcinoma (HCC). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
Nexavar is indicated for the treatment of patients with locally recurrent or metastatic, progressive, differentiated thyroid carcinoma (DTC) that is refractory to radioactive iodine treatment. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
Nexavar is indicated for the treatment of patients with advanced renal cell carcinoma (RCC). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
Palmar-plantar erythrodysesthesia (commonly referred to as hand-foot syndrome) and rash are common adverse effects of sorafenib, occurring in 30 and 40%, respectively, of patients receiving the drug in clinical studies, compared with 7 and 16%, respectively, of patients receiving placebo. Analysis of cumulative event rates suggests that rash and hand-foot syndrome usually are grade 1 or 2 and generally appear during the first 6 weeks of treatment with sorafenib. Management of dermatologic toxicities may include topical therapies for symptomatic relief, temporary interruption of therapy, and/or dosage modification of sorafenib; in severe or persistent cases, permanent discontinuance of sorafenib therapy may be necessary.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1194
Possible increased risk of bleeding. In clinical studies, bleeding (regardless of causality) was reported in 15.3 or 8.2% of patients receiving sorafenib or placebo, respectively. The incidences of grade 3 and 4 bleeding were 2 and 0%, respectively, in patients receiving sorafenib compared with 1.3 and 0.2%, respectively, in patients receiving placebo. Fatal hemorrhage occurred in one patient in each treatment group. Permanent discontinuance of sorafenib should be considered if any bleeding episode requires medical intervention.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1195
GI perforation, sometimes associated with apparent intra-abdominal tumor, has been reported rarely in patients receiving sorafenib. Sorafenib therapy should be discontinued if GI perforation occurs.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1195
Based on its mechanism of action and findings in animals, Nexavar may cause fetal harm when administered to a pregnant woman. Sorafenib caused embryo-fetal toxicities in animals at maternal exposures that were significantly lower than the human exposures at the recommended dose of 400 mg twice daily. Advise women of childbearing potential to avoid becoming pregnant while on Nexavar because of the potential hazard to the fetus.
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
For more Drug Warnings (Complete) data for Sorafenib (16 total), please visit the HSDB record page.
Sorafenib is indicated for the treatment of unresectable hepatocellular carcinoma and advanced renal cell carcinoma.
FDA Label
* Hepatocellular carcinoma:
Nexavar is indicated for the treatment of hepatocellular carcinoma.
* Renal cell carcinoma:
Nexavar is indicated for the treatment of patients with advanced renal cell carcinoma who have failed prior interferon-alpha or interleukin-2 based therapy or are considered unsuitable for such therapy.
* Differentiated thyroid carcinoma:
Nexavar is indicated for the treatment of patients with progressive, locally advanced or metastatic, differentiated (papillary/follicular/Hrthle cell) thyroid carcinoma, refractory to radioactive iodine.
No large changes in QTc interval were observed. After one 28-day treatment cycle, the largest mean QTc interval change of 8.5 ms (upper bound of two-sided 90% confidence interval, 13.3 ms) was observed at 6 hours post-dose on day 1 of cycle 2.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01EX02
L01XE05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX02 - Sorafenib
Absorption
The mean relative bioavailability is 38-49% for the tablet form, when compared to an oral solution. Sorafenib reached peak plasma levels in 3 hours following oral administration. With a high-fat meal, bioavailability is reduced by 29% compared to administration in the fasted state.
Route of Elimination
Following oral administration of a 100 mg dose of a solution formulation of sorafenib, 96% of the dose was recovered within 14 days, with 77% of the dose excreted in feces, and 19% of the dose excreted in urine as glucuronidated metabolites.
Following oral administration of a 100 mg dose of a solution formulation of sorafenib, 96% of the dose was recovered within 14 days, with 77% of the dose excreted in feces and 19% of the dose excreted in urine as glucuronidated metabolites. Unchanged sorafenib, accounting for 51% of the dose, was found in feces but not in urine.
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
After administration of Nexavar tablets, the mean relative bioavailability was 38-49% when compared to an oral solution. Following oral administration, sorafenib reached peak plasma levels in approximately 3 hours. With a moderate-fat meal (30% fat; 700 calories), bioavailability was similar to that in the fasted state. With a high-fat meal (50% fat; 900 calories), bioavailability was reduced by 29% compared to that in the fasted state. It is recommended that Nexavar be administered without food. Mean Cmax and AUC increased less than proportionally beyond oral doses of 400 mg administered twice daily. In vitro binding of sorafenib to human plasma proteins was 99.5%.
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
The absorption and the basic pharmacokinetics following a single dose of sorafenib tosylate were evaluated in female CD-1 mice, male Wistar rats, and female Beagle dogs. For the determination of the absorption of sorafenib in rats, bile duct-cannulated rats (n=5/group) were used. Twenty-four hours after surgery (14)C-sorafenib tosylate was administered orally or intravenously to the rats at a dose of 5 mg/kg sorafenib. The absorption of sorafenib was almost complete in female CD-1 mice (78.6%) and male Wistar rats (79.2%). In Beagle dogs the absorption (67.6 %, calculated from AUC norm values after intravenous and oral administration) and the absolute bioavailability (59.9 %) were lower than in rodents. Maximum plasma concentrations of radioactivity between 1.5 hr and 2 hr after oral administration were observed in all species. After intravenous administration of (14)C-sorafenib tosylate to mice, rats, and dogs the elimination of the radioactivity from plasma occurred with similar terminal half-lives of 6.8, 8.8, and 7.3 hours, respectively. The terminal half-lives of radioactivity after oral administration were 6.1 hours in mice and 5.8 hours in dogs. In rats, terminal half-live after oral administration was longer (11.2 hr) than after intravenous administration. In rats, the elimination of the unchanged compound was slower (half life: 9.3 hr) than in the mice (half life: 6.5 hr) and dogs (half life:4.3 hr). The total plasma clearance in rats was 0.044 L/(hr/kg) corresponding to a blood clearance of 0.049 L/(hr/kg). In mice and dogs the total plasma clearance was 0.13 and 0.15 lL/(hr/kg) respectively. The volume of distribution at steady state ranged from 0.65 l/kg to 0.74 l/kg, depending on the species.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Nexavar, Scientific Discussion p.8-9 (2006). Available from, as of February 21, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000690/WC500027707.pdf
Sorafenib is metabolized primarily in the liver, undergoing oxidative metabolism, mediated by CYP3A4, as well as glucuronidation mediated by UGT1A9. Sorafenib accounts for approximately 70-85% of the circulating analytes in plasma at steady- state. Eight metabolites of sorafenib have been identified, of which five have been detected in plasma. The main circulating metabolite of sorafenib in plasma, the pyridine N-oxide, shows in vitro potency similar to that of sorafenib. This metabolite comprises approximately 9-16% of circulating analytes at steady-state.
Sorafenib undergoes oxidative metabolism by hepatic CYP3A4, as well as glucuronidation by UGT1A9. Inducers of CYP3A4 activity can decrease the systemic exposure of sorafenib. Sorafenib accounted for approximately 70-85% of the circulating analytes in plasma at steady-state. Eight metabolites of sorafenib have been identified, of which 5 have been detected in plasma. The main circulating metabolite of sorafenib, the pyridine N-oxide that comprises approximately 9-16% of circulating analytes at steady-state, showed in vitro potency similar to that of sorafenib.
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
Sorafenib has known human metabolites that include A-D-GlucuronideDISCONTINUED and Sorafenib .
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
25-48 hours
After intravenous administration of (14)C-sorafenib tosylate to mice, rats, and dogs the elimination of the radioactivity from plasma occurred with similar terminal half-lives of 6.8, 8.8, and 7.3 hours, respectively. The terminal half-lives of radioactivity after oral administration were 6.1 hours in mice and 5.8 hours in dogs. In rats, terminal half-live after oral administration was longer (11.2 hr) than after intravenous administration. In rats, the elimination of the unchanged compound was slower (half life: 9.3 hr) than in the mice (half life: 6.5 hr) and dogs (half life:4.3 hr).
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Nexavar, Scientific Discussion p.9 (2006). Available from, as of February 21, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000690/WC500027707.pdf
The mean elimination half-life of sorafenib was approximately 25 to 48 hours.
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0
Sorafenib interacts with multiple intracellular (CRAF, BRAF and mutant BRAF) and cell surface kinases (KIT, FLT-3, VEGFR-2, VEGFR-3, and PDGFR-ß). Several of these kinases are thought to be involved in angiogenesis, thus sorafenib reduces blood flow to the tumor. Sorafenib is unique in targeting the Raf/Mek/Erk pathway. By inhibiting these kinases, genetic transcription involving cell proliferation and angiogenesis is inhibited.
Sorafenib is U.S. Food and Drug Administration-approved for the treatment of renal cell carcinoma and hepatocellular carcinoma and has been combined with numerous other targeted therapies and chemotherapies in the treatment of many cancers. Unfortunately, as with other RAF inhibitors, patients treated with sorafenib have a 5% to 10% rate of developing cutaneous squamous cell carcinoma (cSCC)/keratoacanthomas. Paradoxical activation of extracellular signal-regulated kinase (ERK) in BRAF wild-type cells has been implicated in RAF inhibitor-induced cSCC. Here, /the researchers/ report that sorafenib suppresses UV-induced apoptosis specifically by inhibiting c-jun-NH2-kinase (JNK) activation through the off-target inhibition of leucine zipper and sterile alpha motif-containing kinase (ZAK). Our results implicate suppression of JNK signaling, independent of the ERK pathway, as an additional mechanism of adverse effects of sorafenib. This has broad implications for combination therapies using sorafenib with other modalities that induce apoptosis.
PMID:24170769 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4366425 Vin H et al; Mol Cancer Ther 13 (1): 221-9 (2014)
Several case reports suggest sorafenib exposure and sorafenib-induced hyperbilirubinemia may be related to a (TA)(5/6/7) repeat polymorphism in UGT1A1*28 (UGT, uridine glucuronosyl transferase). We hypothesized that sorafenib inhibits UGT1A1 and individuals carrying UGT1A1*28 and/or UGT1A9 variants experience greater sorafenib exposure and greater increase in sorafenib-induced plasma bilirubin concentration. Inhibition of UGT1A1-mediated bilirubin glucuronidation by sorafenib was assessed in vitro. UGT1A1*28 and UGT1A9*3 genotypes were ascertained with fragment analysis or direct sequencing in 120 cancer patients receiving sorafenib on five different clinical trials. Total bilirubin measurements were collected in prostate cancer patients before receiving sorafenib (n = 41) and 19 to 30 days following treatment and were compared with UGT1A1*28 genotype. Sorafenib exhibited mixed-mode inhibition of UGT1A1-mediated bilirubin glucuronidation (IC(50) = 18 umol/L; K(i) = 11.7 umol/L) in vitro. Five patients carrying UGT1A1*28/*28 (n = 4) or UGT1A9*3/*3 (n = 1) genotypes had first dose, dose-normalized areas under the sorafenib plasma concentration versus time curve (AUC) that were in the 93rd percentile, whereas three patients carrying UGT1A1*28/*28 had AUCs in the bottom quartile of all genotyped patients. The Drug Metabolizing Enzymes and Transporters genotyping platform was applied to DNA obtained from six patients, which revealed the ABCC2-24C>T genotype cosegregated with sorafenib AUC phenotype. Sorafenib exposure was related to plasma bilirubin increases in patients carrying 1 or 2 copies of UGT1A1*28 alleles (n = 12 and n = 5; R(2) = 0.38 and R(2) = 0.77; P = 0.032 and P = 0.051, respectively). UGT1A1*28 carriers showed two distinct phenotypes that could be explained by ABCC2-24C>T genotype and are more likely to experience plasma bilirubin increases following sorafenib if they had high sorafenib exposure. This pilot study indicates that genotype status of UGT1A1, UGT1A9, and ABCC2 and serum bilirubin concentration increases reflect abnormally high AUC in patients treated with sorafenib.
PMID:22307138 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6432766 Peer CJ et al; Clin Cancer Res 18 (7): 2099-107 (2012)
Sorafenib is a kinase inhibitor that decreases tumor cell proliferation in vitro. Sorafenib was shown to inhibit multiple intracellular (c-CRAF, BRAF and mutant BRAF) and cell surface kinases (KIT, FLT- 3, RET, RET/PTC, VEGFR-1, VEGFR- 2, VEGFR- 3, and PDGFR-beta). Several of these kinases are thought to be involved in tumor cell signaling, angiogenesis and apoptosis. Sorafenib inhibited tumor growth of hepatocellular carcinoma (HCC), renal cell carcinoma (RCC), and differentiated thyroid carcinoma (DTC) human tumor xenografts in immunocompromised mice. Reductions in tumor angiogenesis were seen in models of HCC and RCC upon sorafenib treatment, and increases in tumor apoptosis were observed in models of HCC, RCC, and DTC.
US Natl Inst Health; DailyMed. Current Medication Information for NEXAVAR (sorafenib) tablet, film coated (November 2013). Available from, as of February 21, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b50667e4-5ebc-4968-a646-d605058dbef0