


1. 1-(9-deazahypoxanthin-9-yl)-1,4-dideoxy-1,4-iminoribitol
2. Bcx-1777
3. Bcx1777
4. Forodesine
5. Immh Cpd
6. Immucillin H
7. Immucillin-h
1. 284490-13-7
2. Forodesine Hcl
3. Forodesine (hydrochloride)
4. Bcx-1777
5. Bcx-1777 Hydrochloride
6. 6sn82y9u73
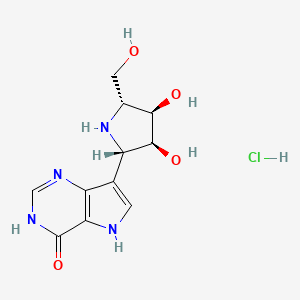
7. (-)-7-((2s,3s,4r,5r)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl)-1,5-dihydro-4h-pyrrolo(3,2-d)pyrimidin-4-one Monohydrochloride
8. 4h-pyrrolo(3,2-d)pyrimidin-4-one, 7-((2s,3s,4r,5r)-3,4-dihydroxy-5-(hydroxymethyl)-2-pyrrolidinyl)-1,5-dihydro-, Monohydrochloride
9. 7-[(2s,3s,4r,5r)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl]-1,5-dihydropyrrolo[3,2-d]pyrimidin-4-one;hydrochloride
10. Immucillin-h Hydrochloride
11. Unii-6sn82y9u73
12. Forodesine Hydrochloride [usan]
13. Forodesine Hydrochloride [usan:jan]
14. Forodesinehydrochloride
15. Chembl550755
16. Schembl1949526
17. Schembl15525350
18. Dtxsid90182647
19. Forodesine Hydrochloride (jan/usan)
20. Forodesine Hydrochloride [mi]
21. Forodesine Hydrochloride [jan]
22. Cs-3780
23. Forodesine Hydrochloride [mart.]
24. Forodesine Hydrochloride [who-dd]
25. Hy-16209
26. D04245
27. Q27265452
28. 7-((2s,3s,4r,5r)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl)-3h-pyrrolo[3,2-d]pyrimidin-4-(5h)-one Hydrochloride
29. 7-[(2s,3s,4r,5r)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl]-3,5-dihydropyrrolo[3,2-d]pyrimidin-4-one;hydrochloride
| Molecular Weight | 302.71 g/mol |
|---|---|
| Molecular Formula | C11H15ClN4O4 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 130 |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 404 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Treatment of Cutaneous T-Cell Lymphoma