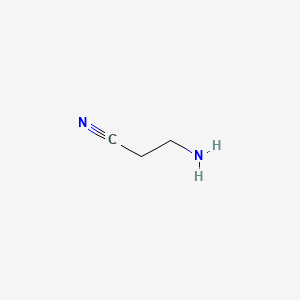

1. Aminopropionitrile
2. Bapn
3. Beta Aminopropionitrile
4. Beta-aminopropionitrile
1. 3-aminopropionitrile
2. 151-18-8
3. 2-cyanoethylamine
4. Aminopropionitrile
5. Beta-aminopropionitrile
6. Bapn
7. 3-aminopropiononitrile
8. Propanenitrile, 3-amino-
9. Beta-cyanoethylamine
10. Beta-alaninenitrile
11. Propionitrile, 3-amino-
12. Beta-aminoethyl Cyanide
13. Beta-alaminenitrile
14. .beta.-aminopropionitrile
15. Nsc 40641
16. 3-amino-propionitrile
17. .beta.-alaninenitrile
18. Chebi:27413
19. .beta.-cyanoethylamine
20. H2nch2ch2cn
21. 38d5lj4kh2
22. Chembl1618272
23. Nsc-40641
24. 3-aminopropionitrile; Aminopropionitrile; Bapn; N-(2-cyanoethyl)amine; Nsc 40641
25. Hsdb 2897
26. Einecs 205-786-0
27. Brn 1698848
28. Cyanoethylamine
29. Unii-38d5lj4kh2
30. Aminoethylcyanide
31. B-alaminenitrile
32. B-alaninenitrile
33. Ccris 8134
34. B-cyanoethylamine
35. 3-aminopropanitrile
36. B-aminoethyl Cyanide
37. Mfcd00014820
38. 3-aminopropanonitrile
39. Beta-aminoproprionitrile
40. N-(2-cyanoethyl)amine
41. Beta-amino Propionitrile
42. Beta-amino-propionitrile
43. Lopac-a-3134
44. .beta.-aminoethyl Cyanide
45. Wln: Z2cn
46. Lopac0_000055
47. Beta-aminopropionitrile Liquid
48. Propanenitrile, 3-amino-, N-c11-13-isoalkyl Derivs.
49. Dtxsid6048418
50. Agspxmvufbbbmo-uhfffaoysa-
51. Aminopropionitrile [hsdb]
52. 3-aminopropionitrile [mi]
53. 3-amino-propionitrile, Aldrichcpr
54. Sodiumbitartrate,monohydrate
55. Cs-d1507
56. Hy-y1750
57. Nsc40641
58. Str02529
59. Zinc1530259
60. Bbl101609
61. Bdbm50232678
62. Propanenitrile, 3-amino-, N-[3-(c12-18-alkyloxy)propyl] Derivs.
63. Stl555405
64. Akos000121388
65. Beta-aminopropionitrile [mart.]
66. Sb75359
67. Ncgc00015048-01
68. Ncgc00015048-02
69. Ncgc00015048-03
70. Ncgc00015048-05
71. Ncgc00162054-01
72. 68130-65-4
73. 68130-66-5
74. 3-aminopropionitrile Stabilized With K2co3
75. Beta-aminopropionitrile [green Book]
76. A0408
77. Ft-0615060
78. 3-aminopropionitrile (stabilized With K2co3)
79. C05670
80. D77355
81. Q3614347
82. W-109080
| Molecular Weight | 70.09 g/mol |
|---|---|
| Molecular Formula | C3H6N2 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 70.053098200 g/mol |
| Monoisotopic Mass | 70.053098200 g/mol |
| Topological Polar Surface Area | 49.8 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 49.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
EXPTL USE: ADMIN OF LYSYL OXIDASE INHIBITOR, BAPN, PREVENTED DEVELOPMENT OF HYPERTENSION & DECR AMT OF VASCULAR COLLAGEN IN RATS IN WHICH HYPERTENSION HAD BEEN INDUCED. HISTOLOGICAL EXAM REVEALED THAT ARTERIOSCLEROTIC CHANGES WERE PREVENTED BY BAPN.
PMID:909131 OOSHIMA A; JPN CIRC J 41(8): 912 (1977)
EXPTL USE: IN YOUNG HYPERTENSIVE RATS, BAPN (20 MG, IP DAILY, FOR 2 WK) PREVENTED DEVELOPMENT OF HYPERTENSION. IN ADULT SPONTANEOUS HYPERTENSIVE RATS (50 MG, IP, DAILY FOR 2 WK) DECR BLOOD PRESSURE.
PMID:723006 OGAWA M, OZAKI M; JPN J PHARMACOL 28(5): 785 (1978)
EXPTL USE: RATS WITH SC IMPLANTED POLYVINYL ALCOHOL SPONGES AND WITH INFLICTED SKIN INCISION WOUNDS RECEIVED A SINGLE INJECTION OF BETA-AMINOPROPIONITRILE (BAPN) AT 4 DOSAGES RANGING FROM 1-40 MG/100 G. EVEN THE LOWEST DOSE OF BAPN INHIBITED LYSYL OXIDASE ACTIVITY FOR 6 HOURS; WITH LARGER DOSAGES THE INHIBITION LASTED LONGER, AT 40 MG BAPN, AT LEAST 48 HOURS. THE MAGNITUDE AND DURATION OF INHIBITION WERE REFLECTED IN THE EXTRACTABILITY OF COLLAGEN AND BURSTING STRENGTH OF THE WOUND. THE DATA SUGGEST THAT A MINIMAL DOSE OF BAPN WOULD BE CLINICALLY EFFECTIVE IF EITHER THE METABOLISM OF THE DRUG WERE REDUCED (BY MONOAMINE OXIDASE INHIBITORS) OR A SUSTAINED-RELEASE PREPARATION OF BAPN WERE USED.
PMID:39201 AREM AJ ET AL; J SURG RES 27(4): 228 (1979)
EXPTL USE: BETA-AMINOPROPIONITRILE (BAPN) WAS TESTED FOR ABILITY TO PREVENT EXCESS COLLAGEN FORMATION IN BLEOMYCIN-INDUCED PULMONARY FIBROSIS IN THE HAMSTER. TWO GROUPS RECEIVED 1 ENDOTRACHEAL DOSE OF BLEOMYCIN; ONE OF THESE WAS INJECTED WITH BAPN TWICE DAILY FOR 30 DAYS. A 3RD GROUP RECEIVED SALINE AND BAPN. THE BLEOMYCIN INCREASED COLLAGEN CONTENT, DECREASED LUNG VOLUME, AND PRODUCED FIBROSIS AND A MORTALITY RATE OF 51%. ADMINISTRATION OF BAPN TO BLEOMYCIN-TREATED ANIMALS PREVENTED EXCESS COLLAGEN ACCUMULATION, PRODUCED LESS FIBROSIS, AND LESSENED MORTALITY RATE TO 24%; BAPN ALONE HAD NO EFFECT ON LUNG MECHANICS OR COLLAGEN CONTENT.
PMID:6175260 RILEY DJ ET AL; AM REV RESPIR DIS 125(1): 67 (1982)
BETA-AMINOPROPIONITRILE (BAPN) WAS FOUND IN URINE WITHIN 1 HR OF ORAL ADMIN. ORAL 250 MG BAPN AT 6 HR INTERVALS EACH DAY FOR 21 DAYS RESULTED IN URINARY BAPN RECOVERIES APPROXIMATING 16% OF TOTAL DOSE. BAPN WAS NOT DETECTED IN SPECIMENS COLLECTED LATER THAN 7 HR AFTER CESSATION OF BAPN DOSAGE. URINARY CYANOACETIC ACID APPEARED MORE SLOWLY THAN BAPN & INCR GRADUALLY TO APPROX 3 TIMES THAT OF URINARY BAPN. AFTER BAPN WAS DISCONTINUED, THERE WAS PROLONGED URINARY EXCRETION OF BAPN-DERIVED CYANOACETIC ACID.
PMID:639425 FLEISHER JH ET AL; CLIN PHARMACOL THER 23(5): 520 (1978)
AFTER APPLICATION TO THE SKIN OF RATS, (14)C-BAPN FREE BASE WAS ABSORBED MORE RAPIDLY AND TO A GREATER EXTENT THAN THE FUMARATE SALT. SIX HOURS AFTER TOPICAL ADMINISTRATION OF THE FREE BASE ONLY TRACES OF (14)C WERE FOUND ON THE SKIN AND LESS THAN 1% OF THE DOSE WITHIN THE SKIN SECTION SUGGESTING RAPID DRUG ABSORPTION.
PMID:6119597 FLEISHER JH ET AL; LIFE SCI 29(24): 2553 (1981)
...BETA-AMINOPROPIONITRILE /IS METABOLIZED/ INTO CYANOACETIC ACID...
The Royal Society of Chemistry. Foreign Compound Metabolism in Mammals. Volume 6: A Review of the Literature Published during 1978 and 1979. London: The Royal Society of Chemistry, 1981., p. 336
BETA-AMINOPROPIONITRILE (BAPN) WAS FOUND IN URINE WITHIN 1 HR OF ORAL ADMIN. ORAL 250 MG BAPN AT 6 HR INTERVALS EACH DAY FOR 21 DAYS RESULTED IN URINARY BAPN RECOVERIES APPROXIMATING 16% OF TOTAL DOSE. BAPN WAS NOT DETECTED IN SPECIMENS COLLECTED LATER THAN 7 HR AFTER CESSATION OF BAPN DOSAGE. URINARY CYANOACETIC ACID APPEARED MORE SLOWLY THAN BAPN & INCR GRADUALLY TO APPROX 3 TIMES THAT OF URINARY BAPN. AFTER BAPN WAS DISCONTINUED, THERE WAS PROLONGED URINARY EXCRETION OF BAPN-DERIVED CYANOACETIC ACID.
PMID:639425 FLEISHER JH ET AL; CLIN PHARMACOL THER 23(5): 520 (1978)
The mechanism of the effect is unknown, but it is thought to be by some action on growth of certain mesodermal tissues. It is not due to one of its major metabolites, cyanoacetic acid, and both the free amino group and the cyano group seem essential for activity. It is not produced if the amino group is in the alpha position, or if in the gamma position in butyronitrile.
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 3156
IT HAS BEEN SUGGESTED THAT LATHYROGENIC AGENTS ACT BY BLOCKING CERTAIN CARBONYL GROUPS NORMALLY PRESENT IN COLLAGEN, & THUS INTERFERING WITH FORMATION OF CROSS LINKAGES. THEIR ACTION MAY BE RETARDED BY RESERPINE OR BY CALCIUM SALTS. /LATHYROGENIC AGENTS/
Clarke, M. L., D. G. Harvey and D. J. Humphreys. Veterinary Toxicology. 2nd ed. London: Bailliere Tindall, 1981., p. 233