

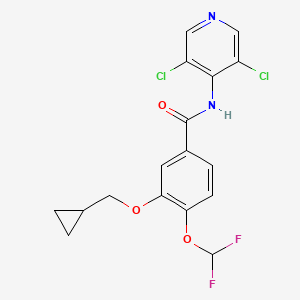

1. 3-cyclopropylmethoxy-4-difluoromethoxy-n-(3,5-di-chloropyrid-4-yl)benzamide
2. Daliresp
1. 162401-32-3
2. Daxas
3. 3-(cyclopropylmethoxy)-n-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide
4. Daliresp
5. By217
6. Byk20869
7. By-217
8. B9302-107
9. By 217
10. 3-(cyclopropylmethoxy)-n-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)benzamide
11. Roflumilast (daxas)
12. Benzamide, 3-(cyclopropylmethoxy)-n-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)-
13. Byk-20869
14. 0p6c6zop5u
15. Chembl193240
16. Chebi:47657
17. 3-cyclopropylmethoxy-n-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide
18. Byk 20869
19. Mfcd00938270
20. B 9302-107
21. Rof
22. Libertek
23. Roflumilast [usan]
24. B-9302-107
25. Roflumilast [usan:inn]
26. Unii-0p6c6zop5u
27. Roflumilastum
28. Roflumilast (jan/usan/inn)
29. Apta-2217
30. 1xmu
31. 1xoq
32. Daliresp (tn)
33. Roflumilast- Bio-x
34. 3g4l
35. Roflumilast [mi]
36. Roflumilast [inn]
37. Roflumilast [jan]
38. 3-cyclopropylmethoxy-4-difluoromethoxy-n-(3,5-di-chloropyrid-4-yl)benzamide
39. Roflumilast [vandf]
40. Roflumilast [mart.]
41. Schembl19158
42. Roflumilast [who-dd]
43. Mls006010074
44. Roflumilast [ema Epar]
45. Gtpl6962
46. Dtxsid8044123
47. Apta 2217
48. Bdbm14774
49. Roflumilast, >=98% (hplc)
50. Amy4219
51. Ex-a059
52. Roflumilast [orange Book]
53. Hms3655p21
54. Hms3748c19
55. Hms3884f09
56. Zinc592419
57. Act02619
58. Bcp03736
59. S2131
60. Arq-151/zoryve (roflumilast Cream)
61. Akos005146309
62. Am84550
63. Ccg-268678
64. Cs-0963
65. Db01656
66. Pb29130
67. Ncgc00346566-01
68. Ncgc00346566-09
69. Ac-23383
70. As-14120
71. Br164364
72. Hy-15455
73. Smr002530074
74. Sy008710
75. Bcp0726000146
76. Ft-0660846
77. R0193
78. Sw220196-1
79. A24672
80. D05744
81. Ab01565852_02
82. 401r323
83. Q693482
84. J-510858
85. Brd-k03194791-001-02-2
86. 3- Cyclo-propylmethoxy-4-difluoromethoxy-n- [3, 5-di-chloropyrid-4-yl]- Benzamid
87. Benzamide, 3-(cyclopropylmethoxy)-n-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)-
88. N-(3,5-dichloropyridin-4-yl)-4-difluoromethoxy-3-cyclopropylmethoxybenzamide
| Molecular Weight | 403.2 g/mol |
|---|---|
| Molecular Formula | C17H14Cl2F2N2O3 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 402.0349540 g/mol |
| Monoisotopic Mass | 402.0349540 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 475 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Daliresp |
| PubMed Health | Roflumilast (By mouth) |
| Drug Classes | Respiratory Agent |
| Drug Label | The active ingredient in DALIRESP tablets is roflumilast. Roflumilast and its active metabolite (roflumilast N-oxide) are selective phosphodiesterase 4 (PDE4) inhibitors. The chemical name of roflumilast is N-(3,5-dichloropyridin-4-yl)-3-cyclopropylm... |
| Active Ingredient | Roflumilast |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mcg |
| Market Status | Prescription |
| Company | Forest Res Inst |
| 2 of 2 | |
|---|---|
| Drug Name | Daliresp |
| PubMed Health | Roflumilast (By mouth) |
| Drug Classes | Respiratory Agent |
| Drug Label | The active ingredient in DALIRESP tablets is roflumilast. Roflumilast and its active metabolite (roflumilast N-oxide) are selective phosphodiesterase 4 (PDE4) inhibitors. The chemical name of roflumilast is N-(3,5-dichloropyridin-4-yl)-3-cyclopropylm... |
| Active Ingredient | Roflumilast |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mcg |
| Market Status | Prescription |
| Company | Forest Res Inst |
Roflumilast is indicated as a treatment to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. Roflumilast is not a bronchodilator and is not indicated for the relief of acute bronchospasm.
FDA Label
Daxas is indicated for maintenance treatment of severe chronic obstructive pulmonary disease (COPD) (FEV1 post-bronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment.
Libertek is indicated for maintenance treatment of severe chronic obstructive pulmonary disease (COPD) (FEV1 post-bronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment.
Daliresp is indicated for maintenance treatment of severe chronic obstructive pulmonary disease (COPD) (FEV1 post-bronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment.
Chronic obstructive pulmonary disease
Roflumilast (and its active metabolite, roflumilast N-oxide) increases cyclic adenosine-3, 5-monophosphate (cAMP) in lung cells by inhibiting PDE4. Increased cAMP activates PKA, which inactivates transcription factors involved in inflammation. Romflumilast also decreases the amount of sputum neutrophils and eosinophils in COPD patients.
R03DX07
R03DX08
R03DX07
R03DX07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03D - Other systemic drugs for obstructive airway diseases
R03DX - Other systemic drugs for obstructive airway diseases
R03DX07 - Roflumilast
Absorption
After a 500mcg dose, the bioavailability of roflumilast is about 80%. In the fasted state, maximum plasma concentrations are reached in 0.5 to 2 hours. While in the fed state, Cmax is reduced by 40%, Tmax is increased by one hour, and total absorption is unchanged.
Route of Elimination
Roflumilast is excreted 70% in the urine as roflumilast N-oxide.
Volume of Distribution
Roflumilast has a Vd of 2.9L/kg with a dose of 500mcg. Permeability of roflumilast across the blood-brain barrier appears to be poor in rat studies.
Clearance
~9.6 L/hour.
Roflumilast is metabolized to roflumilast N-oxide, the active metabolite of roflumilast in humans, by CYP3A4 and CYP1A2.
Plasma half-life of roflumilast is 17 hours and its metabolite is 30 hours (oral dose).
Roflumilast is a phosphodiesterase-4 (PDE-4) inhibitor which, due to its selective inhibition of the PDE4 isoenzyme, has potential antiinflammatory and antimodulatory effects in the pulmonary system. It is thought that the increased levels of intracellular cyclic AMP are responsible for the therapeutic actions of Roflumilast.
