


1. Acid, Phenylethylbarbituric
2. Gardenal
3. Hysteps
4. Luminal
5. Monosodium Salt Phenobarbital
6. Phenemal
7. Phenobarbital
8. Phenobarbital, Monosodium Salt
9. Phenobarbitone
10. Phenylbarbital
11. Phenylethylbarbituric Acid
12. Sodium, Phenobarbital
1. 57-30-7
2. Phenobarbital Sodium Salt
3. Sodium Phenobarbital
4. Sodium Phenobarbitone
5. Luminal Sodium
6. Sodium Luminal
7. Sodium Phenobarbiturate
8. Phenobarbital Sodique
9. Sodium Phenylethylbarbiturate
10. Sodium Phenylethylmalonylurea
11. Sodium 5-ethyl-5-phenylbarbiturate
12. Phenobarbital, Sodium
13. Phenobarbitalum Natricum
14. Sodium 5-ethyl-4,6-dioxo-5-phenyl-1,4,5,6-tetrahydropyrimidin-2-olate
15. 5-ethyl-5-phenylbarbituric Acid Sodium Salt
16. Phenyl-aethyl-barbitursaeure Natrium
17. Sodium 5-ethyl-4,6-dioxo-5-phenyl-1h-pyrimidin-2-olate
18. Phenobarbitone Sodium
19. Phenemalnatrium
20. Phenobal Sodium
21. Phenobarbital Na
22. Sol Phenobarbital
23. Sol Phenobarbitone
24. Phenobarbitalnatrium
25. Phenobarbital Elixir
26. Phenobarbiton-natrium
27. Soluble Phenobarbital
28. Soluble Phenobarbitone
29. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-phenyl-, Monosodium Salt
30. Sodium;5-ethyl-4,6-dioxo-5-phenyl-1h-pyrimidin-2-olate
31. Sw9m9bb5k3
32. Phenobarbitone Sodium Salt
33. Chebi:8070
34. Fenobarbital Sodico
35. Fenobarbital Natrium
36. Ccris 503
37. Fenobarbital Natrium [polish]
38. Fenobarbital Sodico [inn-spanish]
39. Phenobarbital Sodique [inn-french]
40. Einecs 200-322-3
41. Phenobarbitalum Natricum [inn-latin]
42. Unii-sw9m9bb5k3
43. Phenylethylbarbituric Acid, Sodium Salt
44. Phenyl-aethyl-barbitursaeure Natrium [german]
45. Phenobarbital Sodium [usp:inn:jan]
46. Luminal Sodium (tn)
47. 5-ethyl-5-phenyl-2,4,6-(1h,3h,5h)pyrimidinetrione Monosodium Salt
48. Dsstox_cid_1123
49. Dsstox_rid_75954
50. Barbituric Acid, 5-ethyl-5-phenyl-, Sodium Salt
51. Dsstox_gsid_21123
52. Schembl42037
53. Dtxsid0021123
54. Tox21_300271
55. Phenobarbital Sodium (jan/usp/inn)
56. Akos015960550
57. Cas-57-30-7
58. Ncgc00253999-01
59. Ac-11674
60. Db-053032
61. P0890
62. D00701
63. A831383
64. W-105476
65. Q26840934
66. Sodium;5-ethyl-5-phenylpyrimidin-3-ide-2,4,6-trione
67. 5-ethyl-5-phenyl-2,4,6(1h,3h,5h)-pyrimidinetrione Sodium Salt
68. Sodium 5-ethyl-4,6-bis(oxidanylidene)-5-phenyl-1h-pyrimidin-2-olate
| Molecular Weight | 254.22 g/mol |
|---|---|
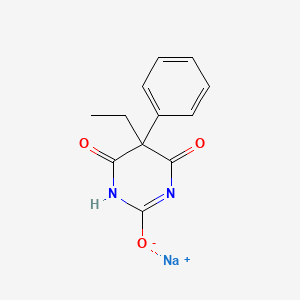
| Molecular Formula | C12H11N2NaO3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 254.06673650 g/mol |
| Monoisotopic Mass | 254.06673650 g/mol |
| Topological Polar Surface Area | 81.6 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 378 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Cytochrome P-450 CYP2B6 Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP2B6. (See all compounds classified as Cytochrome P-450 CYP2B6 Inducers.)
Cytochrome P-450 CYP3A Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inducers.)
Excitatory Amino Acid Antagonists
Drugs that bind to but do not activate excitatory amino acid receptors, thereby blocking the actions of agonists. (See all compounds classified as Excitatory Amino Acid Antagonists.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)

LOOKING FOR A SUPPLIER?