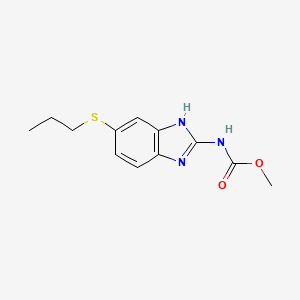

1. Albendazole Monohydrochloride
2. Albendoral
3. Albenza
4. Andazol
5. Bendapar
6. Bilutac
7. Digezanol
8. Disthelm
9. Endoplus
10. Eskazole
11. Gascop
12. Lurdex
13. Mediamix V Disthelm
14. Metiazol
15. Monohydrochloride, Albendazole
16. Sk And F 62979
17. Sk And F-62979
18. Sk And F62979
19. Skf 62979
20. Skf-62979
21. Skf62979
22. Valbazen
23. Zentel
1. 54965-21-8
2. Albenza
3. Eskazole
4. Zentel
5. Valbazen
6. Proftril
7. Albendazolum
8. Andazol
9. Sk&f 62979
10. Methyl 5-(propylthio)-2-benzimidazolecarbamate
11. Skf 62979
12. Sk&f-62979
13. 5-(propylthio)-2-carbomethoxyaminobenzimidazole
14. Skf-62979
15. Methyl N-[6-(propylsulfanyl)-1h-1,3-benzodiazol-2-yl]carbamate
16. Methyl N-(6-propylsulfanyl-1h-benzimidazol-2-yl)carbamate
17. (5-(propylthio)-1h-benzimidazol-2-yl)carbamic Acid Methyl Ester
18. O-methyl N-(5-(propylthio)-2-benzimidazolyl)carbamate
19. Nsc 220008
20. Carbamic Acid, [5-(propylthio)-1h-benzimidazol-2-yl]-, Methyl Ester
21. Carbamic Acid, (5-(propylthio)-1h-benzimidazol-2-yl)-, Methyl Ester
22. Albendazol
23. Bilutac
24. Nsc-220008
25. Chembl1483
26. Methyl [5-(propylsulfanyl)-1h-benzimidazol-2-yl]carbamate
27. Methyl [6-(propylsulfanyl)-1h-benzimidazol-2-yl]carbamate
28. ((propylthio)-5 1h-benzimidazolyl-2) Carbamate De Methyle
29. Chebi:16664
30. Zental
31. F4216019ln
32. Ncgc00016876-01
33. Cas-54965-21-8
34. Dsstox_cid_2563
35. Dsstox_rid_76632
36. Dsstox_gsid_22563
37. Albendazol [inn-spanish]
38. Albendazolum [inn-latin]
39. Methyl [5-(propylthio)-1h-benzimidazol-2-yl]carbamate
40. Methyl 5-(propylthio)-1h-benzo[d]imidazol-2-ylcarbamate
41. Methyl [5-(propylthio)benzimidazol-2-yl]carbamate
42. Methyl N-(5-propylsulfanyl-1h-benzimidazol-2-yl)carbamate
43. Methyl N-[5-(propylsulfanyl)-1h-1,3-benzodiazol-2-yl]carbamate
44. Methyl (nz)-n-(5-propylsulfanyl-1,3-dihydrobenzimidazol-2-ylidene)carbamate
45. Albenza (tn)
46. Hsdb 7444
47. Einecs 259-414-7
48. Mfcd00083232
49. Albendazole (jan/usp/inn)
50. Zenteltrade Mark
51. [5-(propylthio)benzimidazol-2-yl]carbamic Acid Methyl Ester
52. Albenzatrade Mark
53. Andazoltrade Mark
54. Unii-f4216019ln
55. Eskazoletrade Mark
56. Albendazole,(s)
57. ((propylthio)-5 1h-benzimidazolyl-2) Carbamate De Methyle [french]
58. Prestwick_675
59. Albendazole(albenza)
60. Albendazole (albenza)
61. Albendazole [usan:usp:inn:ban:jan]
62. Spectrum_001532
63. Cpd000036735
64. Albendazole [mi]
65. (5-propylsulfanyl-1h-benzoimidazol-2-yl)-carbamic Acid Methyl Ester
66. Prestwick0_000247
67. Prestwick1_000247
68. Prestwick2_000247
69. Prestwick3_000247
70. Spectrum4_000201
71. Spectrum5_001567
72. Albendazole [inn]
73. Albendazole [jan]
74. Chemdivam_000003
75. Chemdiv1_000190
76. Albendazole [hsdb]
77. Albendazole [usan]
78. Albendazole [vandf]
79. Albendazole [mart.]
80. Oprea1_429292
81. Oprea1_585016
82. Oprea1_640007
83. Schembl44682
84. Albendazole [usp-rs]
85. Albendazole [who-dd]
86. Albendazole [who-ip]
87. Bspbio_000034
88. Bspbio_002548
89. Kbiogr_000801
90. Kbioss_002012
91. Mls000069722
92. Albendazolum [who-ip]
93. Bidd:gt0615
94. Divk1c_000704
95. Spectrum1503903
96. Methoxy-n-(5-propylthiobenzimidazol-2-yl)carboxamide
97. Spbio_002253
98. Bpbio1_000038
99. Albendazole [green Book]
100. Dtxsid0022563
101. Hms502d06
102. Hms587i14
103. Hxhwsazorrcqmx-uhfffaoysa-
104. Kbio1_000704
105. Kbio2_002012
106. Kbio2_004580
107. Kbio2_007148
108. Albendazole [orange Book]
109. Ninds_000704
110. Albendazole [ep Monograph]
111. Hms1568b16
112. Hms1922k04
113. Hms2090g19
114. Hms2093k13
115. Hms2095b16
116. Hms2231o03
117. Hms3259b05
118. Hms3369c03
119. Hms3651c15
120. Hms3712b16
121. Pharmakon1600-01503903
122. Albendazole [usp Monograph]
123. [5-(propythio)-1h-benzimidazol-2-yl]carbamic Acid Methyl Ester
124. Bcp12108
125. Hy-b0223
126. Tox21_110659
127. Tox21_302300
128. Ac-015
129. Bbl005883
130. Bdbm50241293
131. Ccg-39620
132. Mfcd01220143
133. N-[6-(propylthio)-1h-benzimidazol-2-yl]carbamic Acid Methyl Ester
134. Nsc220008
135. Nsc758644
136. S1640
137. Stk387550
138. Stl046130
139. Zinc17146904
140. Akos000540882
141. Akos005431684
142. Akos005699352
143. Tox21_110659_1
144. Albendazole 100 Microg/ml In Methanol
145. Ccg-220247
146. Db00518
147. Ks-5159
148. Nc00615
149. Nsc-758644
150. Idi1_000704
151. Methyl [(2z)-5-(propylsulfanyl)-1,3-dihydro-2h-benzimidazol-2-ylidene]carbamate
152. Ncgc00016876-02
153. Ncgc00016876-03
154. Ncgc00016876-04
155. Ncgc00016876-05
156. Ncgc00016876-06
157. Ncgc00016876-07
158. Ncgc00016876-08
159. Ncgc00016876-09
160. Ncgc00016876-10
161. Ncgc00016876-12
162. Ncgc00022896-03
163. Ncgc00022896-04
164. Ncgc00022896-05
165. Ncgc00022896-06
166. Ncgc00022896-07
167. Ncgc00022896-08
168. Ncgc00255250-01
169. Albendazole, Analytical Standard, >=98%
170. Smr000036735
171. Albendazole 100 Microg/ml In Acetonitrile
172. Sbi-0051849.p002
173. Db-052669
174. Ab00052377
175. Ft-0621945
176. Sw196830-3
177. En300-49850
178. Methyl 5-propylthio-2-benzimidazole Carbamate
179. Vu0239747-6
180. Bim-0051849.0001
181. C01779
182. D00134
183. H10782
184. Ab00052377-13
185. Ab00052377-14
186. Ab00052377_15
187. Ab00052377_16
188. A830429
189. Albendazole, Antibiotic For Culture Media Use Only
190. Q411629
191. Sr-01000000171
192. Sr-05000001875
193. Methyl 5-(propylthio)-1h-benzimidazol-2-ylcarbamate
194. Q-200603
195. Q-200604
196. Sr-01000000171-2
197. Sr-05000001875-1
198. Brd-k79131256-001-04-7
199. Brd-k79131256-001-08-8
200. Methyl 5-(propylthio)benzimidazol-2-ylcarbamate
201. Methyl 6-(propylthio)-1h-benzo[d]imidazol-2-ylcarbamate
202. Z1245635850
203. 2-[(methoxycarbonyl)amino]-5-propylthio-1h-benzimidazole
204. Albendazole, European Pharmacopoeia (ep) Reference Standard
205. Methyl (6-(propylthio)-1h-benzo[d]imidazol-2-yl)carbamate
206. Methyl 5-(propylsulfanyl)-1h-benzimidazol-2-ylcarbamate #
207. Albendazole, United States Pharmacopeia (usp) Reference Standard
208. Methyl N-(5-(propylthio)-1h-benzimidazol-2-yl)carbamate
209. Albendazole, Pharmaceutical Secondary Standard; Certified Reference Material
210. Carbamic Acid, N-[5-(propylthio)-1h-benzimidazol-2-yl]-,?methyl Ester
| Molecular Weight | 265.33 g/mol |
|---|---|
| Molecular Formula | C12H15N3O2S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 265.08849790 g/mol |
| Monoisotopic Mass | 265.08849790 g/mol |
| Topological Polar Surface Area | 92.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 291 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Albenza |
| PubMed Health | Albendazole (By mouth) |
| Drug Classes | Anthelmintic |
| Drug Label | ALBENZA (albendazole) is an orally administered broad-spectrum anthelmintic. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is 265.34. It has the following chemical structu... |
| Active Ingredient | Albendazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Amedra Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Albenza |
| PubMed Health | Albendazole (By mouth) |
| Drug Classes | Anthelmintic |
| Drug Label | ALBENZA (albendazole) is an orally administered broad-spectrum anthelmintic. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is 265.34. It has the following chemical structu... |
| Active Ingredient | Albendazole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Amedra Pharms |
Mesh Headings: anthelmintics, anticestodalagents, antiprotozoal agents
National Library of Medicine, SIS; ChemIDplus Record for Albendazole (54965-21-8). Available from, as of April 13, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Albendazole is a benzimidazole carbamate, used for the treatment of gastrointestinal infestations with roundworms, lungworms and tapeworms and adult flukes of Fasciola hepatica.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Albendazole, Summary Report(3). EMEA/MRL/865/03-Final (June 2004). Available from, as of July 25, 2006: https://www.ema.europa.eu/ema/index.jsp?curl=pages/document_library/landing/document_library_search.jsp&murl=menus/document_library/document_library.jsp&mid
Certain microsporidial species that cause intestinal infections in people with AIDS respond partially (Enterocytozoon bieneusi) or completely (Encephalitozoon intestintalis and related Encephalitozoon species) to albendazole; albendazole's sulfoxide metabolite appears to be especially effective against these parasites in vitro.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1126
MEDICATION: ...Used against nematode infections: Ascaris, Necator, Ancylostoma, Trichuris, Enterobius, and systemic nematodes such as Trichinella spiralis, Gnathostoma spinigerum, and larval Angiostrongylus cantonensis. In addition it is used against the larval stages of the cestodes Echinococcus granulosus and E. multilocularis and for treatment of neurocysticercosis caused by Taenia solium.
Ullmann's Encyclopedia of Industrial Chemistry. 6th ed.Vol 1: Federal Republic of Germany: Wiley-VCH Verlag GmbH & Co. 2003 to Present, p. V. 3 185 (2003)
For more Therapeutic Uses (Complete) data for ALBENDAZOLE (21 total), please visit the HSDB record page.
Leukopenia has occurred in less than 1% of patients receiving albendazole, and rarely, granulocytopenia, pancytopenia, agranulocytosis, or thrombocytopenia have been reported. Therefore, blood counts should be performed at the start of, and every 2 weeks during, each 28-day treatment cycle. The manufacturer states that if decreases in the total leukocyte count occur, treatment with albendazole may be continued if the decreases are modest and do not progress.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 46
Because albendazole has been associated with mild to moderate increases of hepatic enzymes in about 16% of patients receiving the drug in clinical trials, and may cause hepatotoxicity, liver function tests should be performed prior to each course of albendazole therapy and at least every 2 weeks during treatment with the drug. If clinically important increases in liver function test results occur, albendazole should be discontinued. The drug can be reinstituted when liver enzymes return to pretreatment levels, but laboratory tests should be performed frequently during repeat therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 46
Albendazole may cause harm to the fetus and should be used during pregnancy only if the benefits justify the risk to the fetus and only in clinical circumstances where no alternative management is appropriate. Women of childbearing age should begin treatment only after a negative pregnancy test, and should be cautioned against becoming pregnant while receiving albendazole or within 1 month of completing treatment with the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 46
Teratogenic in animals (rats and rabbits) and is not recommended in pregnancy or in infants younger than 2 years.
Haddad, L.M. (Ed). Clinical Management of Poisoning and Drug Overdose 3rd Edition. Saunders, Philadelphia, PA. 1998., p. 714
For more Drug Warnings (Complete) data for ALBENDAZOLE (9 total), please visit the HSDB record page.
For the treatment of parenchymal neurocysticercosis due to active lesions caused by larval forms of the pork tapeworm, Taenia solium and for the treatment of cystic hydatid disease of the liver, lung, and peritoneum, caused by the larval form of the dog tapeworm, Echinococcus granulosus.
Albendazole is a broad-spectrum anthelmintic. The principal mode of action for albendazole is by its inhibitory effect on tubulin polymerization which results in the loss of cytoplasmic microtubules.
Anthelmintics
Agents that kill parasitic worms. They are used therapeutically in the treatment of HELMINTHIASIS in man and animal. (See all compounds classified as Anthelmintics.)
Anticestodal Agents
Agents used to treat tapeworm infestations in man or animals. (See all compounds classified as Anticestodal Agents.)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
P - Antiparasitic products, insecticides and repellents
P02 - Anthelmintics
P02C - Antinematodal agents
P02CA - Benzimidazole derivatives
P02CA03 - Albendazole
Absorption
Poorly absorbed from the gastrointestinal tract due to its low aqueous solubility. Oral bioavailability appears to be enhanced when coadministered with a fatty meal (estimated fat content 40 g)
Route of Elimination
Albendazole is rapidly converted in the liver to the primary metabolite, albendazole sulfoxide, which is further metabolized to albendazole sulfone and other primary oxidative metabolites that have been identified in human urine. Urinary excretion of albendazole sulfoxide is a minor elimination pathway with less than 1% of the dose recovered in the urine. Biliary elimination presumably accounts for a portion of the elimination as evidenced by biliary concentrations of albendazole sulfoxide similar to those achieved in plasma.
Albendazole is variably and erractically absorbed after oral admin; absorption is enhanced by the presence of fatty foods and possibly by bile salts as well. After a 400-mg oral dose, albendazole cannot be detected in plasma, because the drug is rapidly metabolized in the liver and possibly in the intestine as well, to albendazole sulfoxide, which has potent anthelmintic activity. Both the (+) and (-) enantiomers of albendazole sulfoxide are formed, but in human beings the (+) enantiomer reaches much higher peak concn in plasma and is cleared much more slowly than the (-) form. Total sulfoxide attains peak plasma concn of about 300 ng/mL, but with wide interindividual variation. Albendazole sulfoxide is about 70% bound to plasma proteins ... It is well distributed into various tissues, including hydatid cysts, where it reaches a concn of about one-fifth that in plasma ... Both sulfoxide derivatives are oxidized further to the nonchiral sulfone metabolite of albendazole, which is pharmacologically inactive; this reaction favors the (-) sulfoxide and probably becomes rate limiting in determining the clearance ... Albendazole metabolites are excreted mainly in the urine.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1126
Oral bioavailability of albendazole appears to be increased when the drug is administered with a fatty meal; when the drug is administered with meals containing about 40 g of fat, plasma concentrations of albendazole sulfoxide are up to 5 times higher than those observed when the drug is administered to fasting patients
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 46
Sheep bearing permanent ruminal and abomasal cannulae were given a single oral dose of 10 mg/kg bw albendazole as a 2.5% formulation. Albendazole was absorbed unchanged from the rumen. Once in the body it was rapidly degraded, and sulfone metabolites were detected in plasma, the former achieving the greater level. All 3 compounds were present in the abomasum. Presumably albendazole was passed through the stomachs while the metabolites were secreted or diffused into this organ. Non-detectable levels of all 3 compounds were reached in plasma and rumen at 96 hr and in abomasum at 120 hr.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 25: Toxicological Evaluation of Ceratin Veterinary Drug Residues in Food: Albendazole (1990). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v25je01.htm
The parent compound was virtually undetectable in the plasma of Sprague Dawley males and females given a single gavage dose of 10.6 mg/kg bw albendazole in an aqueous suspension. Rapid metabolism let to the appearance of the sulfoxide and subsequently the sulfone derivatives in plasma. Both metabolites decreased to very low levels at 18 hr. Daily dosing at 10.6 mg/kg bw in males for a period of 10 days resulted in lower plasma levels of sulfoxide and higher levels of the sulfone. Albendazole induces certain hepatic drug-metabolising enzymes, which may be responsible for enhancing the degradation of sulfoxide to sulfone following repeated administration.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 25: Toxicological Evaluation of Ceratin Veterinary Drug Residues in Food: Albendazole (1990). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v25je01.htm
For more Absorption, Distribution and Excretion (Complete) data for ALBENDAZOLE (9 total), please visit the HSDB record page.
Hepatic. Rapidly converted in the liver to the primary metabolite, albendazole sulfoxide, which is further metabolized to albendazole sulfone and other primary oxidative metabolites that have been identified in human urine.
Albendazole is converted first to a sulfoxide and then to a sulfone. All of these reactions are catalyzed by flavin monooxygenases (FMO) and/or cytochrome P450. Both enzymes are efficient catalysts of S-oxygenation ...
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 176
Albendazole is metabolized in the liver to an active metabolite, albendazole sulfoxide, which accounts for detectable plasma concentrations of the drug; systemic anthelmintic activity of the drug has been attributed to this metabolite.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 47
Albendazole ... is rapidly metabolized in the liver and possibly in the intestine as well, to albendazole sulfoxide, which has potent anthelmintic activity. Both the (+) and (-) enantiomers of albendazole sulfoxide are formed, but in human beings the (+) enantiomer reaches much higher peak concn in plasma and is cleared much more slowly than the (-) form. Total sulfoxide attains peak plasma concn of about 300 ng/mL, but with wide interindividual variation. Albendazole sulfoxide is about 70% bound to plasma proteins and has a highly variable plasma half-life ranging from about 4 to 15 hr. It is well distributed into various tissues, including hydatid cysts, where it reaches a concn of about one-fifth that in plasma. This probably explains why albendazole is more effective than mebendazole for treating hydatid cyst disease. Formation of albendazole sulfoxide is catalyzed by both microsomal flavin monooxygenase and isoforms of cytochrome P450 in the liver and possibly also in the intestine. Hepatic flavin monooxygenase activity appears associated with (+) albendazole sulfoxide formation, whereas cytochromes P450 preferentially produce the (-) sulfoxide metabolite. Both sulfoxide derivatives are oxidized further to the nonchiral sulfone metabolite of albendazole, which is pharmacologically inactive; this reaction favors the (-) sulfoxide and probably becomes rate limiting in determining the clearance and plasma half-life of the bioactive (+) sulfoxide metabolite. Induction of enzymes involved in sulfone formation from the (+) sulfoxide could account for some of the wide variation noted in plasma half-lives of albendazole sulfoxide. Indeed, in animal models, benzimidazoles can induce their own metabolism. Albendazole metabolites are excreted mainly in the urine.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1126
Sheep bearing permanent ruminal and abomasal cannulae were given a single oral dose of 10 mg/kg bw albendazole as a 2.5% formulation. Albendazole was absorbed unchanged from the rumen. Once in the body it was rapidly degraded, and sulfone metabolites were detected in plasma, the former achieving the greater level. All 3 compounds were present in the abomasum. Presumably albendazole was passed through the stomachs while the metabolites were secreted or diffused into this organ. Non-detectable levels of all 3 compounds were reached in plasma and rumen at 96 hr and in abomasum at 120 hr.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 25: Toxicological Evaluation of Ceratin Veterinary Drug Residues in Food: Albendazole (1990). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v25je01.htm
For more Metabolism/Metabolites (Complete) data for ALBENDAZOLE (12 total), please visit the HSDB record page.
Albendazole has known human metabolites that include Albendazole oxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Terminal elimination half-life ranges from 8 to 12 hours (single dose, 400mg).
Albendazole ... is rapidly metabolized ... to albendazole sulfoxide, which ... has a highly variable plasma half-life ranging from about 4 to 15 hr ... Both /(+) and (-)/ sulfoxide derivatives are oxidized further to the nonchiral sulfone metabolite ... This reaction favors the (-) sulfoxide and probably becomes rate limiting in determining ... plasma half-life of the bioactive (+) sulfoxide metabolite. Induction of enzymes involved in sulfone formation from the (+) sulfoxide could account for some of the wide variation noted in plasma half-lives of albendazole sulfoxide.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1126
Albendazole causes degenerative alterations in the tegument and intestinal cells of the worm by diminishing its energy production, ultimately leading to immobilization and death of the parasite. It works by binding to the colchicine-sensitive site of tubulin, thus inhibiting its polymerization or assembly into microtubules. As cytoplasmic microtubules are critical in promoting glucose uptake in larval and adult stages of the susceptible parasites, the glycogen stores of the parasites are depleted. Degenerative changes in the endoplasmic reticulum, the mitochondria of the germinal layer, and the subsequent release of lysosomes result in decreased production of adenosine triphosphate (ATP), which is the energy required for the survival of the helminth.
Benzimidazoles produce many biochemical changes in susceptible nematodes, eg, inhibition of mitochondrial fumarate reductase, reduced glucose transport, and uncoupling of oxidative phosphorylation ... /but/ the primary action ... /should be/ to inhibit microtubule polymerization by binding to beta-tubulin. The selective toxicity of these agents derives from the fact that specific, high-affinity binding to parasite beta-tubulin occurs at much lower concn than does binding to the mammalian protein ... Benzimidazole-resistant Haemonchus contortus display reduced high-affinity drug binding to beta-tubulin and alterations in beta-tubulin isotype gene expression that correlate with drug resistance ... Two identified mechanisms of drug resistance in nematodes involve both a progressive loss of "susceptible" beta-tubulin gene isotypes together with emergence of a "resistant" isotype with a conserved point mutation that encodes a tyrosine instead of phenylalanine at position 200 of beta-tubulin. While this mutation may not be required for benzimidazole resistance in all parasites, eg, Giardia lamblia, benzimidazole resistance in parasitic nematodes is unlikely to be overcome by novel benzimidazole analogs, because tyrosine also is present at position 200 of human beta-tubulin. /Benzimidazoles/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1126
Although the exact mechanism of action of albendazole has not been fully elucidated, the principal anthelmintic effect of benzimidazoles, including albendazole, appears to be the specific, high-affinity binding of the drug to free beta-tubulin in parasite cells, resulting in selective inhibition of parasite microtubule polymerization, and inhibition of microtubule-dependent uptake of glucose. Benzimidazole drugs bind to the beta-tubulin of parasites at much lower concentrations than to mammalian beta-tubulin protein; the drugs do not inhibit glucose uptake in mammals, and do not appear to have any effect on blood glucose concentrations in humans
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 47
The mode of action of albendazole is by binding strongly with the tubulin in the cells of nematodes. The intestinal cells of the nematode are particularly affected, resulting in a loss of absorptive function which causes the nematodes to starve to death.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Albendazole, Summary Report(3). EMEA/MRL/865/03-Final (June 2004). Available from, as of July 25, 2006: https://www.ema.europa.eu/ema/index.jsp?curl=pages/document_library/landing/document_library_search.jsp&murl=menus/document_library/document_library.jsp&mid