


1. 50910-55-9
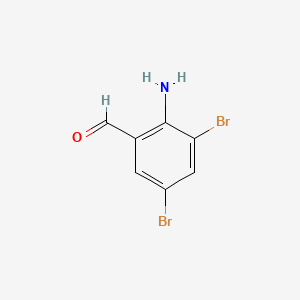
2. 2-amino-3,5-dibromo-benzaldehyde
3. 3,5-dibromo-2-aminobenzaldehyde
4. 3,5-dibromoanthranilaldehyde
5. Benzaldehyde, 2-amino-3,5-dibromo-
6. Mfcd00671100
7. Bromhexine Impurity B
8. Einecs 256-841-0
9. Bromhexine Ep Impurity B
10. Ec 256-841-0
11. Schembl285148
12. Zinc57067
13. Dtxsid70198943
14. Amy16465
15. Bcp06911
16. Stl450950
17. Akos015854696
18. 2-amino-3,5-dibromobenzaldehyde, 97%
19. Ac-10733
20. Ac-31356
21. As-12042
22. Bp-12420
23. Sy038949
24. Db-020382
25. Cs-0030731
26. D2625
27. Ft-0611038
28. F12175
29. A828361
30. W-105925
31. Ambroxol Ep Impurity E; 2-amino-3,5-dibromobenzaldehyde
| Molecular Weight | 278.93 g/mol |
|---|---|
| Molecular Formula | C7H5Br2NO |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 278.87174 g/mol |
| Monoisotopic Mass | 276.87379 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 153 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
2-Amino-3,5-dibromobenzaldehyde is a known human metabolite of ambroxol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560