


1. 58258-01-8
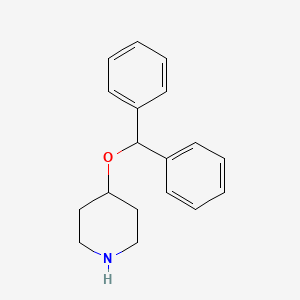
2. 4-(benzhydryloxy)piperidine
3. 4-benzhydryloxypiperidine
4. 4-benzhydryloxy-piperidine
5. Desalkylebastine
6. Desalkyl Ebastine
7. Piperidine, 4-(diphenylmethoxy)-
8. Z2qv6qd5vv
9. 4-(4-benzhydryloxy)piperidine
10. 4-(diphenylmethoxy)-piperidine
11. Desalkyl Ebastine-[d5]
12. Unii-z2qv6qd5vv
13. 4-diphenylmethoxypiperidine
14. 4-diphenylmethoxy-piperidine
15. Schembl1802062
16. Dtxsid00388512
17. Mfcd06659472
18. Zinc22056059
19. Akos015910915
20. Sb37794
21. Db-053175
22. Cs-0342012
23. Ft-0640517
24. C16027
25. A831806
| Molecular Weight | 267.4 g/mol |
|---|---|
| Molecular Formula | C18H21NO |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 267.162314293 g/mol |
| Monoisotopic Mass | 267.162314293 g/mol |
| Topological Polar Surface Area | 21.3 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 245 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
4-(4-tert-butylphenyl)-4-oxobutanal is a known human metabolite of ebastine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560