

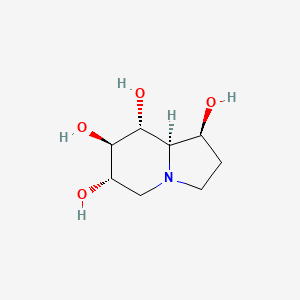

1. 1,6,7,8-tetrahydroxyoctahydroindolizine
2. 1-epicastanospermine
3. 6,7-diepicastanospermine
4. 6-epicastanospermine
5. Castinospermine
1. 79831-76-8
2. Castinospermine
3. 6-epicastanospermine
4. 1,6,7,8-tetrahydroxyoctahydroindolizine
5. (1s,6s,7r,8r,8ar)-1,6,7,8-tetrahydroxyindolizidine
6. 6,7-diepicastanospermine
7. (1s,6s,7r,8r,8ar)-1,2,3,5,6,7,8,8a-octahydroindolizine-1,6,7,8-tetrol
8. Chebi:27860
9. Q0i3184xm7
10. 79831-76-8 (free Base)
11. Nsc-625381
12. (1s,6s,7r,8r,8ar)-octahydroindolizine-1,6,7,8-tetrol
13. (1s,6s,7r,8r,8ar)-octahydroindolizine-1,6,7,8-tetraol
14. (1s-(1alpha,6beta,7alpha,8beta,8alphabeta))-octahydro-1,6,7,8-indolizinetetrol
15. Cts
16. Sr-01000597675
17. Mfcd00017555
18. Nsc 625381
19. Unii-q0i3184xm7
20. Indolizine Der.
21. Nsc625381
22. 2cbu
23. Octahydro-indolizine-1,6,7,8-tetraol
24. Cid54445
25. Cast
26. (1s,6s,7r,8r,8ar)-octahydro-1,6,7,8-indolizinetetrol
27. Molmap_000013
28. Upcmld-dp122
29. Castanospermine [mi]
30. Schembl61040
31. Bspbio_001552
32. Kbiogr_000272
33. Kbioss_000272
34. Mls000028641
35. Chembl311226
36. Upcmld-dp122:001
37. Upcmld-dp122:002
38. Upcmld-dp122:003
39. Bcbcmap01_000133
40. Bdbm36388
41. Kbio2_000272
42. Kbio2_002840
43. Kbio2_005408
44. Kbio3_000543
45. Kbio3_000544
46. Bio1_000396
47. Bio1_000885
48. Bio1_001374
49. Bio2_000272
50. Bio2_000752
51. Dtxsid601026043
52. Hms1361n14
53. Hms1791n14
54. Hms1989n14
55. Hms2232g11
56. Hms3266b08
57. Hms3402n14
58. Hms3414h05
59. Hms3678h05
60. Hy-n2022
61. Zinc3775177
62. Akos024458634
63. Ccg-208170
64. Cs-5633
65. Db01816
66. Idi1_034022
67. Smp1_000059
68. Ncgc00024773-02
69. Ncgc00024773-04
70. Smr000059227
71. B6439
72. Octahydro-indolizine-1,6,7,8-tetraol, 13
73. C02256
74. Q753104
75. Sr-01000597675-1
76. Sr-01000597675-4
77. W-203822
78. Castanospermine From Castanospermum Australe Seeds
79. 81041b9a-d148-44dc-a86d-c78d6abf9633
80. (1s,6s,7r,8r,8ar)-1,6,7,8-tetrahydroxyindolizidine, 98%
81. 1,6,7,8-indolizinetetrol, Octahydro-,?(1s,6s,7r,8r,8ar)-
82. [1s-(1?,6?,7?,8?,8a?)]-octahydro-1,6,7,8-indolizinetetrol
83. Castanospermine, >=94% (gc), Bioultra, From Castanospermum Australe Seeds
84. 1,6,7,8-indolizinetetrol, Octahydro-, (1s-(1alpha,6beta,7alpha,8beta,8abeta))-
| Molecular Weight | 189.21 g/mol |
|---|---|
| Molecular Formula | C8H15NO4 |
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 189.10010796 g/mol |
| Monoisotopic Mass | 189.10010796 g/mol |
| Topological Polar Surface Area | 84.2 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 201 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Glycoside Hydrolase Inhibitors
Compounds that inhibit or block the activity of GLYCOSIDE HYDROLASES such as ALPHA-AMYLASES and ALPHA-GLUCOSIDASES. (See all compounds classified as Glycoside Hydrolase Inhibitors.)
