

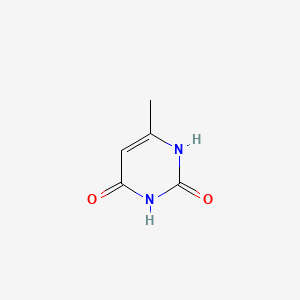

1. 6-methyluracil, 14c-labeled
2. Awd 23-15
3. Awd-23-15
4. Methacil
5. Methyluracil
6. Pseudothymine
1. 626-48-2
2. 2,4-dihydroxy-6-methylpyrimidine
3. 6-methylpyrimidine-2,4(1h,3h)-dione
4. Pseudothymine
5. 4-methyluracil
6. 6-methyl-1h-pyrimidine-2,4-dione
7. 6-methyl-2,4(1h,3h)-pyrimidinedione
8. 6-methylpyrimidine-2,4-diol
9. 2,4(1h,3h)-pyrimidinedione, 6-methyl-
10. Uracil, 6-methyl-
11. 2,4-pyrimidinediol, 6-methyl-
12. 2(1h)-pyrimidinone, 4-hydroxy-6-methyl-
13. Nsc 9456
14. Awd 23-15
15. Mfcd00006028
16. Chebi:74733
17. Chembl1650614
18. 5o052w0g6i
19. Nsc-9456
20. 6-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione
21. Pseudothymine (van)
22. Hsdb 5508
23. Einecs 210-949-4
24. 24pyrimidinedione6methyl
25. Metacyl
26. Methacyl
27. Unii-5o052w0g6i
28. Ai3-25472
29. 4-(6)-methyluracil
30. Methyluracil, 4-
31. Wln: T6mvmvj F1
32. Dsstox_cid_30880
33. Dsstox_gsid_52308
34. Schembl60308
35. 6-methyluracil [mi]
36. 4-methyl-2.6-dioxypyrimidin
37. Methyluracil [who-dd]
38. 6-methylpyrimidine-2,4-dione
39. 6-methyluracil [hsdb]
40. Dtxsid8052308
41. Schembl21990343
42. 6-methyluracil, 97% (hplc)
43. Nsc9456
44. Hms1762e01
45. Zinc162484
46. 2,4-dihydroxy-6-methyl-pyrimidine
47. Act07641
48. Albb-022469
49. Bcp26951
50. Hy-y1125
51. Str00504
52. 2,3h)-pyrimidinedione, 6-methyl-
53. 2,4-dihydroxyl-6-methyl Pyrimidine
54. Tox21_304049
55. Bdbm50106396
56. Stl283926
57. Stl426163
58. 6-methyl-2,4(1h,3h)pyrimidinedione
59. Akos000120980
60. Akos002303990
61. Ac-2536
62. Am81337
63. Sb57748
64. Ncgc00357256-01
65. Bp-12331
66. Cas-626-48-2
67. Sy001859
68. 6-methyl-2,4(1h,3h)-pyrimidinedione #
69. Db-016105
70. Bb 0256780
71. Cs-0017143
72. Ft-0610121
73. M0454
74. A15712
75. D70607
76. 4-hydroxy-6-methyl-1,2-dihydro-pyrimidin-2-one
77. Ab-323/25048156
78. 1-boc-3-(2-fluoro-benzylamino)-piperidine
79. Q-200556
80. Q4161980
81. Z56871945
82. F0322-0043
83. 6mu
| Molecular Weight | 126.11 g/mol |
|---|---|
| Molecular Formula | C5H6N2O2 |
| XLogP3 | -0.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 126.042927438 g/mol |
| Monoisotopic Mass | 126.042927438 g/mol |
| Topological Polar Surface Area | 58.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 195 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-ulcer Agents; Radiation-protective Agents; Adjuvants, Immunologic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999
EXPTL USE: 4-METHYLURACIL (50 MG/KG/DAY) SHOWED SIGNIFICANT ANTIMETASTATIC EFFECTS WHEN ADMIN ORALLY TO RATS WITH LYMPHO- OR LYMPHOHEMATOGENIC METASTASES OF PLISS LYMPHOSARCOMA OR WALKER CARCINOSARCOMA FOR 3 DAYS BEFORE & AFTER LAPAROTOMY.
YAREMENKO KV; PREVENTION OF THE METASTATIC SPREAD OF TRANSPLANTED TUMORS BY PREPARATIONS WITH ANABOLIC PROPERTIES UNDER SURGICAL STRESS CONDITIONS; ONKOLOGIYA (KIEV) 4: 20 (1973)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Radiation-Protective Agents
Drugs used to protect against ionizing radiation. They are usually of interest for use in radiation therapy but have been considered for other purposes, e.g. military. (See all compounds classified as Radiation-Protective Agents.)
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
UNCHANGED 6-METHYL-2-THIOURACIL (46% OF DOSE), 6-METHYLURACIL (9%), 6-METHYL-2-METHYLTHIOURACIL (2%), 6-METHYL-4-OXOPYRIMIDINE (2%), 2-AMINO-6-METHYL-4-OXOPYRIMIDINE (0.2%), & UREA (1%) WERE EXCRETED IN URINE OF RATS THAT HAD BEEN TREATED ORALLY WITH 6-METHYL-2-THIOURACIL.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 4: A Review of the Literature Published during 1974 and 1975. London: The Chemical Society, 1977., p. 153
...STUDIES INDICATED THE FORMATION OF FOUR VOLATILE SUBSTANCES AFTER IRRADIATION OF A 10 PPM AQ BROMACIL SOLN FOR 6 DAYS. THE MAJOR PRODUCT (37%) WAS 6-METHYLURACIL.
Menzie, C.M. Metabolism of Pesticides, Update II. U.S. Department of the Interior, Fish Wildlife Service, Special Scientific Report - Wildlife No. 2l2. Washington, DC: U.S. Government Printing Office, 1978., p. 288
A MIXED CULTURE OF PSEUDOMONAS SPECIES & PROACTINOMYCES RUBER UTILIZED 6-METHYLURACIL AS THE SOLE SOURCE OF CARBON & NITROGEN. 6-METHYLURACIL WAS OXIDATIVELY CONVERTED TO URACIL WHICH WAS SUBSEQUENTLY METABOLIZED TO BARBITURIC ACID & UREA.
ZVYAGINTSEVA IS, MAMULINA NS; OXIDATIVE METABOLISM OF 4 6-METHYLURACIL; IZV AKAD NAUK USSR, SER BIOL (6) 929 (1969)