

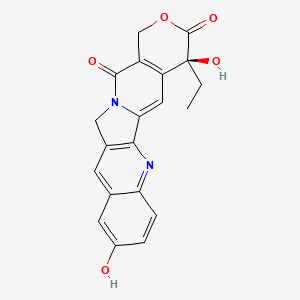

1. 10-hydroxycamptothecine
1. 19685-09-7
2. (s)-10-hydroxycamptothecin
3. Hydroxycamptothecin
4. 10-hydroxycamptothecine
5. 10-hydroxy Camptothecin
6. Hydroxycamptothecine
7. (s)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
8. Camptothecin, Hydroxy-
9. 10-hcpt
10. 10-hydroxy-camptothecin
11. (4s)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
12. Camptothecine, 10-hydroxy-
13. Irinotecan Related Compound A
14. Nsc107124
15. Nsc-107124
16. Chembl273862
17. Chebi:81395
18. 9z01632krv
19. Mfcd02093100
20. (+)-(s)-10-hydroxycamptothecin
21. (s)-4-ethyl-4,9-dihydroxy-1,12-dihydro-14h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h)-dione
22. Camptothecin, 10-hydroxy-
23. (20s)-4-ethyl-4,9-dihydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
24. (s)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione ((s)-10-hydroxycamptothecin)
25. Nsc 107124
26. (s)-10-hydroxycamptothecin Hydrate
27. Unii-9z01632krv
28. (20s)-10-hydroxycamptothecin
29. Camptothecin, 10-hydroxy
30. 10-hydroxy-cpt
31. Spectrum_001639
32. Specplus_000763
33. Spectrum2_001660
34. Spectrum3_001621
35. Spectrum4_001815
36. Spectrum5_000549
37. Ethyl(dihydroxy)[?]dione
38. Schembl25875
39. Bspbio_003281
40. Kbiogr_002454
41. Kbioss_002119
42. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4-ethyl-4,9-dihydroxy-, Hydrate, (s)-
43. Divk1c_006859
44. Spectrum1504123
45. Spbio_001819
46. Kbio1_001803
47. Kbio2_002119
48. Kbio2_004687
49. Kbio2_007255
50. Kbio3_002501
51. Dtxsid00941444
52. Ex-a988
53. Bcp01385
54. Hy-n0095
55. Zinc3979155
56. (+)-10-hydroxycamptothecin
57. Bdbm50008922
58. Ccg-38770
59. S2423
60. S3898
61. Akos015919293
62. Ac-5502
63. Bcp9000058
64. Cs-5193
65. Db12385
66. 10-hydroxycamptothecin [who-dd]
67. Ncgc00095986-01
68. Ncgc00095986-02
69. Ncgc00095986-03
70. Ncgc00095986-04
71. Ncgc00178165-01
72. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione-,4-ethyl-4,9-dihydroxy-, (s)-
73. Ac-13221
74. As-13196
75. Nci60_000173
76. Sy010687
77. H1463
78. N2591
79. 85h097
80. A25382
81. C17939
82. Irinotecan Related Compound A [usp-rs]
83. Sr-05000002620
84. Q-100241
85. Sr-05000002620-1
86. Brd-k63784565-001-02-1
87. Brd-k63784565-001-03-9
88. Q27155328
89. 4-ethyl-4,9-dihydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
90. (19s)-19-ethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2(11),3,5,7,9,15(20)-heptaene-14,18-dione
91. (alphas)-alpha,2-dihydroxy-alpha-ethyl-8-(hydroxymethyl)-9-oxo-9,11-dihydroindolizino[1,2-b]quinoline-7-acetic Acid 7,8-lactone
92. (s)-10-hydroxycamptothecin;-;(+/-)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
93. (s)-4-ethyl-4,9-dihydroxy-1h-pyrano[3 Inverted Exclamation Mark ,4 Inverted Exclamation Mark :6,7]indolizino[1,2-b]quinoline-3,14-(4h,12h)-dione
94. (s)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-(4h,12h)-dione
95. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4-ethyl-4,9-dihydroxy-, (4s)-
96. 1h-pyrano[3',7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione-,4-ethyl-4,9-dihydroxy-, (s)-
97. 4-ethyl-4,10-dihydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
98. 4-ethyl-4,9-dihydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (10-hydroxycamptothecin)
1. Hcpt
2. Hydroxycamptothecin
3. Hydroxycamptothecine
| Molecular Weight | 364.4 g/mol |
|---|---|
| Molecular Formula | C20H16N2O5 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 364.10592162 g/mol |
| Monoisotopic Mass | 364.10592162 g/mol |
| Topological Polar Surface Area | 100 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 774 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)