
1. 1,2-cyclohexanediamine, (1r,2r)-
2. 1,2-cyclohexanediamine, (1r-trans)-
3. 1,2-cyclohexanediamine, (1s,2s)-
4. 1,2-cyclohexanediamine, (1s-trans)-
5. 1,2-cyclohexanediamine, (cis)-isomer
6. 1,2-cyclohexanediamine, (trans)-(r)-isomer
7. 1,2-cyclohexanediamine, (trans)-(s)-isomer
8. 1,2-cyclohexanediamine, (trans)-isomer
9. 1,2-dach
10. 1,2-diaminocyclohexane
11. 1,2-diaminocyclohexane, (+)-
12. 1,2-diaminocyclohexane, (-)-
13. 1,2-diaminocyclohexane, Cis-
14. 1,2-diaminocyclohexane, Trans-
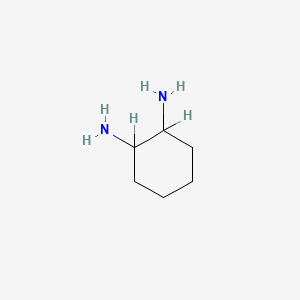
15. Cyclohexane-1,2-diamine
16. Dach
17. Meso-1,2-diaminocyclohexane
18. Trans-1,2-cyclohexanediamine
19. Trans-1,2-diaminocyclohexane
1. Cyclohexane-1,2-diamine
2. 1,2-diaminocyclohexane
3. 694-83-7
4. Cyclohex-1,2-ylenediamine
5. (rel)-oxaliplatin
6. Dtxsid0027301
7. Dtxcid707301
8. 1,2-cylohexanediamine
9. Cas-694-83-7
10. Dach
11. Cis-1,2-cyclohexandiamine
12. Hsdb 5748
13. (r,r)-dach
14. Trans-1,2-cyclohexaneiamine
15. Einecs 211-776-7
16. Unii-c82tx76bhh
17. Brn 0506142
18. Pyridoxal Phosphate (calcium Salt)
19. (+)-s,s-1,2-diaminocyclohexane
20. Mfcd00062985
21. Rac-(1r,2r)-cyclohexane-1,2-diamine
22. (1r,2s)-1,2-diaminocyclohexane; (r)(s)-1,2-diaminocyclohexane; (+/-)-cis-cyclohexane-1,2-diamine; Cis-(1s,2r)-1,2-diaminocyclohexane; Cis-1,2-cyclohexanediamine; Cis-1,2-diaminocyclohexane
23. 1,2-diamino Cyclohexane
24. Cyclohexane 1,2-diamine
25. Ec 211-776-7
26. 1,2-diaminocyclohexane,c&t
27. C82tx76bhh
28. Schembl29457
29. 0-13-00-00001 (beilstein Handbook Reference)
30. Schembl687020
31. Pyridoxal, 5-(dihydrogen Phosphate), Calcium Salt (1:1) (8ci)
32. Dch-99
33. Chembl1356279
34. Chebi:93778
35. Bcp04075
36. Bcp22662
37. Tox21_201883
38. Tox21_303220
39. Bbl027344
40. Mfcd00001491
41. Nsc362640
42. Nsc362641
43. Stl372729
44. (+/-)-1,2-trans-diaminocyclohexane
45. Akos000119859
46. Akos016842498
47. Nsc-362640
48. Nsc-362641
49. Pb47571
50. Sb44188
51. Cyclohexanediamine1,2-cyclohexanediamine
52. Ncgc00166039-01
53. Ncgc00166039-02
54. Ncgc00257130-01
55. Ncgc00259432-01
56. Sy001269
57. Sy004140
58. Vs-08529
59. (1r,2r)-(-)- 1,2-diaminocyclohexane
60. Db-010810
61. Db-011652
62. Db-027311
63. Db-042698
64. Am20070551
65. D0277
66. Ns00002024
67. En300-19626
68. (1s)-trans-1,2-cyclohexanediamine; (s,s)-dach
69. L000760
70. W-104620
71. Q27165475
72. Z104474510
73. (' Inverted Exclamation Marka)-trans-1,2-diaminocyclohexan
74. (1r,2r)-cyclohexane-1,2-diamine;(1r,2r)-(-)-1,2-cyclohexanediamine
75. 1224727-05-2
| Molecular Weight | 114.19 g/mol |
|---|---|
| Molecular Formula | C6H14N2 |
| XLogP3 | -0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 52 |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 62.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MALONATO-, HYDROXYMALONATO-, DINITRO-, HYDROXONITRATO-, & SULFATO(1,2-DIAMINOCYCLOHEXANE) PLATINUM(II), PREPARED FROM DICHLORO(1,2-DIAMINOCYCLOHEXANE WERE MORE EFFECTIVE THAN DICHLORO (1,2-DIAMINOCYCLOHEXANE)PLATINUM(II) IN THE TREATMENT OF L1210 LEUKEMIA IN MICE, BOTH ALONE & IN COMBINATION WITH CYCLOPHOSPHAMIDE OR YOSHI 864.
GALE GR, MEISCHEN SJ; 1,2-DIAMINOCYCLOHEXANE PLATINUM(II) COMPLEXES HAVING ANTINEOPLASTIC ACTIVITY; US PATENT APPLICATION PATENT NUMBER 719689 9/02/76 (US DEPT OF HEALTH, EDUCATION & WELFARE)
/PLATINUM CO-ORDINATION COMPLEXES/ SHARE THE COMMON FORMULA PTA2X2... THE ACTIVE LIGANDS ARE MONODENTATE ANIONS OF INTERMEDIATE LEAVING ABILITY, WHEREAS THE AMINE A LIGANDS INFLUENCE THE SOLUBILITY OF THE COMPLEX. THE STEREOSPECIFICITY OF THE ANTI-TUMOR EFFECT IS AN IMPORTANT PROPERTY OF THE PLATINUM COMPLEXES. ... INDIRECT EVIDENCE SUGGESTS THAT BINDING TO ADJACENT GUANINE BASES IN THE SAME DNA STRAND IS IMPORTANT TO THE MODE OF ACTION. AFTER INITIAL BINDING TO GUANINE BASES, DNA BECOMES LOCALLY DENATURED AND EXPOSES ADDITIONAL CROSS-LINKING SITES. /PLATINUM COMPLEXES/
The Royal Society of Chemistry. Foreign Compound Metabolism in Mammals. Volume 6: A Review of the Literature Published during 1978 and 1979. London: The Royal Society of Chemistry, 1981., p. 364
...THE TOXIC EFFECT ON ANIMALS OF PLATINUM ITSELF DIFFERS ESSENTIALLY FROM THAT OF THE COMPLEX SALTS. ...THE ACTION OF THESE SALTS IS NOT DUE TO THE SEPARATE COMPONENTS, PLATINUM AND THE BASE, BUT TO THAT OF THE MOLECULE AS A WHOLE ON SENSITIVE TISSUES, AND THEIR PHYSIOLOGICAL EFFECT, THOUGH VARYING IN DEGREE, IS IDENTICAL IN CHARACTER. ...AN INCREASE IN THE VALENCY OF THE AMMONIUM COMPONENT WAS ASSOCIATED WITH A STRONGER "CURARE-LIKE" ACTION WHILE A CHANGE IN THE VALENCY OF THE PLATINUM HAD NO INFLUENCE ON THE TOXIC EFFECT. /PLATINUM COMPLEXES/
Browning, E. Toxicity of Industrial Metals. 2nd ed. New York: Appleton-Century-Crofts, 1969., p. 271