


1. Hki 272
2. Hki-272
3. Hki272
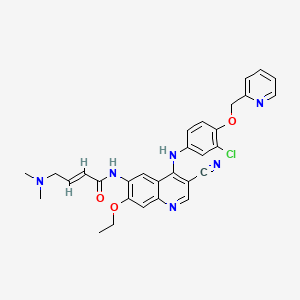
4. N-(4-(3-chloro-4-(2-pyridinylmethoxy)anilino)-3-cyano-7-ethoxy-6-quinolyl)-4-(dimethylamino)-2-butenamide
5. Neratinib Maleate
6. Nerlynx
1. 698387-09-6
2. Hki-272
3. Neratinib (hki-272)
4. Nerlynx
5. Hki 272
6. Pb-272
7. Jjh94r3pwb
8. Chembl180022
9. (2e)-n-[4-[[3-chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide
10. Cdp-820
11. 698387-09-6 (free Base)
12. Hki272
13. Way-179272
14. (2e)-n-(4-{[3-chloro-4-(pyridin-2-ylmethoxy)phenyl]amino}-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide
15. (2e)-n-(4-((3-chloro-4-((pyridin-2-yl)methoxy)phenyl)amino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide
16. (e)-n-(4-((3-chloro-4-(pyridin-2-ylmethoxy)phenyl)amino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide
17. N-(4-(3-chloro-4-(2-pyridinylmethoxy)anilino)-3-cyano-7-ethoxy-6-quinolyl)-4-(dimethylamino)-2-butenamide
18. 2-butenamide, N-(4-((3-chloro-4-(2-pyridinylmethoxy)phenyl)amino)-3-cyano-7-ethoxy-6-quinolinyl)-4-(dimethylamino)-, (2e)-
19. Neratinib [usan]
20. Neratinib(hki-272)
21. (e)-n-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide
22. Neratinib [usan:inn]
23. Unii-jjh94r3pwb
24. Neratinib- Bio-x
25. (e)-n-[4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide
26. Pb 272
27. Neratinib [inn]
28. Neratinib [mi]
29. Neratinib (usan/inn)
30. Neratinib - Hki-272
31. Neratinib [mart.]
32. Neratinib [who-dd]
33. Schembl571762
34. Schembl571763
35. Gtpl5686
36. Chebi:61397
37. Amy9255
38. Dtxsid70220132
39. Ex-a062
40. Bcpp000151
41. Zinc3916214
42. Bdbm50161957
43. Mfcd09752958
44. Nsc757439
45. Nsc800803
46. S2150
47. Way-179272-b
48. Akos005146340
49. Akos025149637
50. Bcp9000984
51. Ccg-270036
52. Db11828
53. Nsc-757439
54. Nsc-800803
55. Ncgc00241101-01
56. Ncgc00241101-03
57. Ncgc00241101-09
58. Ac-25073
59. As-16279
60. Bn164645
61. Hy-32721
62. N1062
63. Ec-000.2260
64. A25338
65. D08950
66. 387n096
67. Q-101402
68. Q6995920
69. Brd-k85606544-001-01-8
70. (2e)-n-(4-((3-chloro-4-((pyridin-2-yl)methoxy)phenyl)amino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide Neratinib
71. (2e)-n-[4-[[3-chloro-4-(2-pyridinylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-2-butenamide
72. (e)-4-dimethylamino-but-2-enoic Acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-phenylamino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide
73. 4-dimethylamino-but-2-enoic Acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-phenylamino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide
74. Hki-272; Pb272;;(2e)-n-[4-[[3-chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide;hki-272
75. N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide
76. N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)butanamide
| Molecular Weight | 557.0 g/mol |
|---|---|
| Molecular Formula | C30H29ClN6O3 |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Exact Mass | 556.1989665 g/mol |
| Monoisotopic Mass | 556.1989665 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 881 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | NERLYNX |
| Active Ingredient | NERATINIB MALEATE |
| Company | PUMA BIOTECH (Application Number: N208051. Patents: 6288082, 7399865, 7982043, 8518446, 8790708, 9139558, 9211291, 9630946) |
For use as an extended adjuvant treatment in adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy.
FDA Label
Nerlynx is indicated for the extended adjuvant treatment of adult patients with early stage hormone receptor positive HER2-overexpressed/amplified breast cancer and who are less than one year from the completion of prior adjuvant trastuzumab based therapy.
Neratinib is a tyrosine kinase inhibitor which exhibits antitumor action against Epidermal Growth Factor Receptor (EGFR), HER2, and Human Epidermal Growth Factor Receptor 4 (HER4) postive carcinomas.
L01EH02
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EH - Human epidermal growth factor receptor 2 (her2) tyrosine kinase inhibitors
L01EH02 - Neratinib
Absorption
Neratinib and its major active metabolites M3. M6, and M7 have a Tmax of 2-8 h. Administration with a high fat meal increases Cmax by 1.7-fold and total exposure by 2.2-fold. Administration with a standard meal increases Cmax by 1.2-fold and total exposure by 1.1-fold. Administration with gastric acid reducing agents such as proton pump inhibitors reduces Cmax by 71% and total exposure by 65%.
Route of Elimination
97.1% of the total dose is excreted in the feces and 1.13% in the urine.
Volume of Distribution
The apparent volume of distribution at steady state is 6433 L.
Clearance
The total clearance during multiple doses is 216 L/h for after the first dose and 281 L/h during steady state.
Neratinib is mainly undergoes metabolism via CYP3A4. It is also metabolized by flavin-containing monooxygenase to a lesser extent. The systemic exposures of neratinib's active metabolites M3, M6, M7, and M11 are 15%, 33%, 22%, and 4%.
The mean half life of elimination ranges from 7-17 h following a single dose. The mean plasma half life during multiple doses is 14.6 h for neratinib, 21.6 h for M3, 13.8 h for M6, and 10.4 h for M7.
Neratinib binds to and irreversibly inhibits EGFR, HER2, and HER4. This prevents auotphoshorylation of tyrosine residues on the receptor and reduces oncogenic signalling through the mitogen-activated protein kinase and Akt pathways.