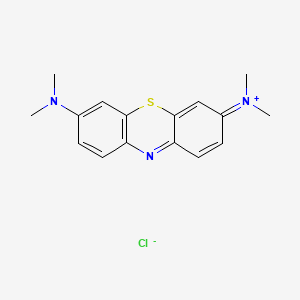

1. Basic Blue 9
2. Blue 9, Basic
3. Blue N, Methylene
4. Blue, Methylene
5. Blue, Swiss
6. Blue, Urolene
7. Chromosmon
8. Methylene Blue N
9. Methylthionine Chloride
10. Methylthioninium Chloride
11. Swiss Blue
12. Urolene Blue
1. 61-73-4
2. Basic Blue 9
3. Methylthioninium Chloride
4. Solvent Blue 8
5. Swiss Blue
6. Chromosmon
7. Methylene Blue Anhydrous
8. 3,7-bis(dimethylamino)phenothiazin-5-ium Chloride
9. Methylene Blue N
10. Methylene Blue Bb
11. C.i. Basic Blue 9
12. Methylenium Ceruleum
13. Tetramethylthionine Chloride
14. Bleu De Methylene
15. Methylene Blue Chloride
16. Methylthionine Chloride
17. External Blue 1
18. Methylene Blue A
19. Methylene Blue B
20. Methylene Blue D
21. Methylene Blue G
22. Calcozine Blue Zf
23. Methylene Blue Bd
24. Methylene Blue Bp
25. Methylene Blue Bx
26. Methylene Blue Bz
27. Methylene Blue Fz
28. Methylene Blue Gz
29. Methylene Blue Nz
30. Methylene Blue Sg
31. Methylene Blue Sp
32. Methylene Blue Zf
33. Methylene Blue Zx
34. Tetramethylene Blue
35. Methylene Blue 2b
36. Methylene Blue Bba
37. Methylene Blue Bpc
38. Methylene Blue Hgg
39. Methylene Blue Iad
40. Methylene Blue Jfa
41. Sandocryl Blue Brl
42. Methylenblau
43. Methylene Blue 2bf
44. Methylene Blue 2bn
45. Methylene Blue 2bp
46. M-b Tabs
47. Rember
48. Mitsui Methylene Blue
49. Methylthionium Chloride
50. Leather Pure Blue Hb
51. Modr Methylenova
52. Schultz No. 1038
53. Aizen Methylene Blue Bh
54. Aizen Methylene Blue Fz
55. Methylene Blue Polychrome
56. Methylene Blue Zinc Free
57. C.i. 52015
58. Yamamoto Methylene Blue B
59. D And C Blue Number 1
60. Methylene Blue (medicinal)
61. Phenothiazin-5-ium, 3,7-bis(dimethylamino)-, Chloride
62. Yamamoto Methylene Blue Zf
63. Ext D And C Blue No. 1
64. Methylene Blue I (medicinal)
65. Cloruro De Metiltioninio
66. Methylene Blue Nf (medicinal)
67. Methylthioninii Chloridum
68. Hidaco Methylene Blue Salt Free
69. Methylene Blue Bb (zinc Free)
70. Methylene Blue Usp (medicinal)
71. Lowacryl Blue 9
72. Chlorure De Methylthioninium
73. Methylene Blue Usp Xii (medicinal)
74. 3,7-bis(dimethylamino)phenazathionium Chloride
75. Methylene Blue Chloride (biological Stain)
76. X 138
77. Mfcd00012111
78. 12262-49-6
79. Ci 52015
80. Ci-52015
81. 8nap7826ub
82. Chebi:6872
83. 105504-42-5
84. Methylthioninium Chloride [inn]
85. [7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium;chloride
86. Urolene Blue
87. Provayblue
88. Nsc-215213
89. Nsc-617593
90. Azul De Metileno
91. Dsstox_cid_3296
92. N-[7-(dimethylamino)-3h-phenothiazin-3-ylidene]-n-methylmethanaminium Chloride
93. Ceruleum Methylenum
94. Dsstox_rid_76963
95. Dsstox_gsid_23296
96. Methylenum Coeruleum
97. Ci Basic Blue 9
98. Zinc Free Methylene Blue
99. Caswell No. 567
100. Methylenblau [german]
101. Methylthionini Chloridum
102. 3h-phenothiazine, 3-methochloride
103. Trx0014
104. Phenothiazin-5-ium, 3,7-bis(dimethylamino)-, Chloride (1:1)
105. Modr Methylenova [czech]
106. Chembl405110
107. Modr Zasadita 9
108. Modr Zasadita 9 [czech]
109. Cas-61-73-4
110. N-(7-(dimethylamino)-3h-phenothiazin-3-ylidene)-n-methylmethanaminium Chloride
111. Nsc3089
112. 152071-32-4
113. Ccris 833
114. Metiltioninio Cloruro [dcit]
115. Metiltioninio Cloruro
116. Modr Rozpoustedlova 8 [czech]
117. 3h-phenothiazine, 7-(dimethylamino)-3-(methylimino)-, 3-methochloride
118. Modr Rozpoustedlova 8
119. Nsc215213
120. Nsc617593
121. C.i. 52 015
122. Hsdb 1405
123. Ncgc00167496-01
124. Ncgc00167496-03
125. Phenothiazin-5-ium,7-bis(dimethylamino)-, Chloride
126. Einecs 200-515-2
127. Methylthioninii Chloridum [inn-latin]
128. Epa Pesticide Chemical Code 039505
129. Nsc 215213
130. Cloruro De Metiltioninio [inn-spanish]
131. Unii-8nap7826ub
132. Wln: T C666 Bs Ey Inj Euk1&1 Mn1&1 &g &421
133. Chlorure De Methylthioninium [inn-french]
134. Methylene Blue Usp
135. Methylene Azure Ii
136. Prestwick_326
137. Methylene Blue (inhibitor)
138. Schembl1351
139. Methylene Blue [mi]
140. Mls000719838
141. Methylene Blue [hsdb]
142. Basic Blue 9 [inci]
143. Dog (couch) Grass Powder
144. Dtxsid0023296
145. Hms2598o03
146. Pharmakon1600-01505444
147. Ci No 52015
148. Nsc-3089
149. Trx-0014
150. Tox21_112497
151. Tox21_302087
152. Nsc759135
153. S4535
154. Stk018918
155. Akos000486124
156. Akos015916406
157. Tox21_112497_1
158. Ccg-267696
159. Db09241
160. 4-(1-hydroxy-ethyl)-benzoicacid
161. Methylthioninium Chloride [mart.]
162. Ncgc00255351-01
163. Ac-15225
164. As-35256
165. Basic Blue 9;tetramethylthionine Chloride
166. Hy-14536
167. Methylene Blue, 1% W/v Aqueous Solution
168. Methylthioninium Chloride [who-dd]
169. Methylthioninium Chloride Proveblue
170. Smr000304367
171. Sy076497
172. Methylthioninium Chloride (methylene Blue)
173. Methylene Blue (c.i. 52015) Biochemica
174. Methylthioninium Chloride [ema Epar]
175. A0574
176. Ft-0622580
177. M0501
178. M2392
179. C00220
180. M-3598
181. Methylthioninium Chloride [ep Impurity]
182. 3,7-bis(dimethylamino)-5-phenothiazinium Chloride
183. Q422134
184. Methylthioninium Chloride Anhydrous [who-ip]
185. 3,7-bis(dimethylamino)-phenothiazin-5-ium Chloride Salt
186. Methylthioninii Chloridum Anhydrous [who-ip Latin]
187. Methylene Blue Solution (methanol Solution) [for Cell Staining]
188. 3-n,3-n,7-n,7-n-tetramethylphenothiazin-5-ium-3,7-diamine;chloride
189. N-(7-(dimethylamino)-3h-phenothiazin-3-ylidene)-n-methylmethanaminiumchloride
190. 1341-90-8
191. 6476-03-5
192. 97130-83-1
| Molecular Weight | 319.9 g/mol |
|---|---|
| Molecular Formula | C16H18ClN3S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 319.0909965 g/mol |
| Monoisotopic Mass | 319.0909965 g/mol |
| Topological Polar Surface Area | 43.9 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 483 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Enzyme Inhibitors
National Library of Medicine's Medical Subject Headings. Methylene Blue. Online file (MeSH, 2018). Available from, as of August 29, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Methylene blue is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 29, 2018: https://clinicaltrials.gov/
Drug-induced methemoglobinemia. /Included in US product label/
NIH; DailyMed. Current Medication Information for Methylene blue injection (Updated: November 20, 2017). Available from, as of September 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fde64824-2be5-4d85-8d57-5098ca6890bb
Methylene blue posses weak urinary antiseptic properties. Methylene blue directly inhibits calcium binding by oxalate and by organic stone matrix. The drug also acts as a crystal poison at the interface, reducing the tendency of calcium oxalate particles to aggregate. In addition, it reverses intracellular acidosis (such as that in renal tubule acidosis), apparently by competing with diphosphopyridine nucleotide as a hydrogen receptor.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
For more Therapeutic Uses (Complete) data for Methylene blue (32 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: SEROTONIN SYNDROME WITH CONCOMITANT USE OF SEROTONERGIC DRUGS. Methylene Blue Injection may cause serious or fatal serotonergic syndrome when used in combination with serotonergic drugs. Avoid concomitant use of Methylene blue injection with selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors.
NIH; DailyMed. Current Medication Information for Methylene blue injection (Updated: November 20, 2017). Available from, as of September 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fde64824-2be5-4d85-8d57-5098ca6890bb
Large IV doses of methylene blue may produce nausea, vomiting, abdominal pain, precordial pain, dizziness, headache, profuse sweating, dyspnea, hypertension, and mental confusion. Urinary tract irritation may occur. High IV dosage or high local concentrations of methylene blue may cause formation of methemoglobin and cyanosis.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Hemolysis and hemolytic anemia may occur, especially in young infants and patients with glucose-6-phosphate dehydrogenase (G-6-PD) deficiency.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Hypersensitivity, manifested as wheal and flare reactions at the injection site, has been reported. Severe hypersensitivity reactions, including anaphylaxis, generalized urticaria, and hypotension, tachycardia, and bronchospasm, have been reported following local instillation or injection of methylene blue.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
For more Drug Warnings (Complete) data for Methylene blue (20 total), please visit the HSDB record page.
Indicated for the treatment of pediatric and adult patients with acquired methemoglobinemia. Other clinical applications of methylene blue include improvement of hypotension associated with various clinical states, an antiseptic in urinary tract infections, treatment of hypoxia and hyperdynamic circulation in cirrhosis of liver and severe hepatopulmonary syndrome, and treatment of ifofosamide induced neurotoxicity.
Acute symptomatic treatment of medicinal and chemical products- induced methaemoglobinaemia.
Methylthioninium chloride Proveblue is indicated in adults, children and adolescents (aged 0 to 17 years old).
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
V03AB17
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB17 - Methylthioninium chloride
V - Various
V04 - Diagnostic agents
V04C - Other diagnostic agents
V04CG - Tests for gastric secretion
V04CG05 - Methylthioninium chloride
Route of Elimination
Excreted in urine and bile. About 75% of an oral dose excreted in urine, primarily as stabilized colorless leukomethylene blue.
Volume of Distribution
10 mg/kg (in rats).
Clearance
3.00.7 L/min.
... The concentration of methylene blue in whole blood was measured using high-performance liquid chromatography in seven volunteers after IV and oral administration of 100 mg methylene blue with and without mesna. The distribution of methylene blue in different tissues was measured in rats after intraduodenal and IV application. The time course of methylene blue in whole blood after IV administration showed a multiphasic time course with an estimated terminal half-life of 5.25 hr. Following oral administration, the area under the concentration-time curve was much lower (9 nmol/min/mL vs 137 nmol/min/mL). Co-administration of mesna, which could influence distribution by ion-pairing, did not alter the pharmacokinetics. The urinary excretion of methylene blue and its leukoform was only moderately higher after IV administration (18% vs 28% dose). Intraduodenal administration to rats resulted in higher concentrations in intestinal wall and liver but lower concentrations in whole blood and brain than IV methylene blue. Differences in organ distribution of methylene blue are mainly responsible for the different pharmacokinetics after oral and IV administration. ...
PMID:10952480 Peter C et al; Eur J Clin Pharmacol 56 (3): 247-50 (2000)
Methylene blue is well absorbed from the GI tract, and peak plasma concentrations occur approximately 1-2 hours after an oral dose. ... Following distribution into tissues, methylene blue is rapidly reduced to leukomethylene blue (leucomethylthioninium chloride). Metabolism to leucomethylene blue may be less efficient in neonates than in older individuals. Methylene blue is excreted in urine and bile. About 75% of an oral dose of methylene blue is excreted in urine, mostly as stabilized colorless leukomethylene blue. On exposure to air, the urine turns green or blue, due to the presence of the oxidation product methylene azure (methylene blue sulfone). Some unchanged drug is also excreted in urine.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
BACKGROUND: Although blue dye is routinely used for lymphatic mapping, it is not used for lymphatic mapping in pregnancy-associated breast cancer, because of concern of fetal risk. METHODS: To investigate the safety of blue dye for lymphatic mapping in pregnant women, the pharmacokinetics of methylene blue dye were examined in 10 nonpregnant women, and the results were extrapolated to estimate maximal fetal exposure to the dye. RESULTS: Plasma and urine measurements indicated that the dye quickly distributed from the breast injection site to the circulation, with 32% of the total dose excreted in urine within 48 hours. Combined with existing data on organ distribution of methylene blue, the estimated maximal dose to the fetus is 0.25 mg (5% of the administered dose), likely further reduced by other physiologic factors related to pregnancy. CONCLUSIONS: The analysis suggests that methylene blue dye can be used for lymphatic mapping in pregnancy-associated breast cancer with minimal fetal risk.
PMID:21167367 Pruthi S et al; Am J Surg 201 (1): 70-5 (2011)
The disposition and urinary excretion pharmacokinetics of methylene blue were determined after its intravenous administration at 15 mg/kg to mature female sheep. Comparisons were made between methylene blue administered alone or subsequent to 50 mg/kg sodium nitrite. The overall elimination rate constant (beta) of methylene blue, 0.0076 +/- 0.0016 min-1, was not influenced by prior administration of sodium nitrite. However, the distribution rate was significantly altered by sodium nitrite. Very little of the methylene blue was eliminated in the urine either intact or as leukomethylene blue in spite of its relatively short half life. ...
PMID:6492250 Burrows GE; J Vet Pharmacol Ther 7 (3): 225-31(1984)
Following distribution into tissues, rapidly reduced to leukomethylene blue (leucomethylthioninium chloride). Metabolism to leucomethylene blue may be less efficient in neonates than in older individuals.
Methylene blue can be reduced to a colorless form, leukomethylene blue; together, these compounds form a reversible oxidation-reduction system. In low concentrations, methylene blue accelerates conversion of methemoglobin to hemoglobin. In patients with methemoglobinemia, methylene blue is reduced to leukomethylene blue by methemoglobin reductases in erythrocytes; leukomethylene blue then reduces methemoglobin to hemoglobin. In high concentrations, methylene blue oxidizes the ferrous iron of reduced hemoglobin to the ferric state, thereby changing hemoglobin to methemoglobin.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Following distribution into tissues, methylene blue is rapidly reduced to leukomethylene blue (leucomethylthioninium chloride). Metabolism to leucomethylene blue may be less efficient in neonates than in older individuals.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
56.5 hours (after IV dose).
Following IV administration, the estimated half-life of methylene blue is 5-6.5 hours.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
... The concentration of methylene blue in whole blood was measured using high-performance liquid chromatography in seven volunteers after IV and oral administration of 100 mg methylene blue with and without mesna. The distribution of methylene blue in different tissues was measured in rats after intraduodenal and IV application. The time course of methylene blue in whole blood after IV administration showed a multiphasic time course with an estimated terminal half-life of 5.25 hr. ...
PMID:10952480 Peter C et al; Eur J Clin Pharmacol 56 (3): 247-50 (2000)
* Main mechanism of action involves inhibition of nitric oxide synthase and guanylate cyclase. * In Alzheimers Disease: a mechanistic study found that methylene blue oxidizes cysteine sulfhydryl groups on tau to keep tau monomeric. One preclinical treatment study in tauopathy mice reported anti-inflammatory or neuroprotective effects mediated by the Nrf2/antioxidant response element (ARE); another reported insoluble tau reduction and a learning and memory benefit when given early. * In Methemoglobinemia: Methylene Blue acts by reacting within RBC to form leukomethylene blue, which is a reducing agent of oxidized hemoglobin converting the ferric ion (fe+++) back to its oxygen-carrying ferrous state(fe++). * As antimalarial agent: Methylene Blue, a specific inhibitor of P.falciparum glutathione reductase has the potential to reverse CQ resistance and it prevents the polymerization of haem into haemozoin similar to 4-amino-quinoline antimalarials. * For ifosfamide induced neurotoxicity: Methylene blue functions as an alternate electron acceptor. It acts to reverse the NADH inhibition caused by gluconeogenesis in the liver while blocking the transformation of chloroethylamine into chloroacetaldehyde. In addition, it inhibits various amine oxidase activities, which also prevents the formation of chloroacetaldehyde.
The mechanism of modulation of cyclic guanosine monophosphate accumulation by methylene blue, a putative inhibitor of soluble guanylate cyclase, was investigated in cultured rabbit pulmonary arterial smooth muscle cells. Control or methylene blue pretreated rabbit pulmonary arterial smooth muscle were stimulated with sodium nitroprusside, nitrosothiols or endothelium derived relaxing factor released basally from bovine pulmonary arterial endothelial cells, in short term cocultures. The putative endothelium-derived relaxing factor, S-nitroso-L-cysteine, a stab1e deaminated analog of S-nitroso-L-cysteine, S-nitroso-3-mercaptoproprionic acid and sodium nitroprusside produced concentration-dependent (1-100 uM) increase (1.5- to 12-fold) in rabbit pulmonary arterial smooth muscle cells cyclic guanosine monophospate levels. Methylene blue pretreatment inhibited S-nitroso-L-cysteine and sodium nitroprusside induced cyclic guanosine monophosphate accumulation by 51% to 100%, but S-nitroso-3-mercaptoproprionic acid mediated responses were not altered by methylene blue. The inhibition profile of methylene blue on nitrovasodilator induced cyclic guanosine monophosphate accumulation was quantitatively reproduced by extracellular generation of superoxide anion with xanthine (100 uM) and xanthine oxidase (5 mU). Similarly to methylene blue pretreatment, superoxide anion generation had no effects on base-line cyclic guanosine phosphate levels or cyclic guanosine phosphate responses elicited by S-nitroso-3-mercaptoproprionic acid. Furthermore, methylene blue induced a dose and time dependent generation of superoxide anion from rabbit pulmonary arterial smooth muscle cells, as evidenced from spectrophotometric determination of cytochrome c reduction. Inhibition of cyclic guanosine monophosphate accumulation in response to S-nitroso-L-cysteine and sodium nitroprusside by methylene blue was completely prevented by superoxide dismutase but not catalase. Selective pretreatment of endothelial cells with methylene blue before co-culture with untreated rabbit pulmonary arterial smooth muscle produced a reduction in rabbit pulmonary arterial smooth muscle cyclic guanosine monophosphate levels of a magnitude comparable with that seen in cocultures of methylene blue pretreated rabbit pulmonary arterial smooth muscle with untreated endothelial cells, and which was partially prevented by superoxide dismutase.
PMID:1328604 Marczin N et al; J Pharmacol Exp Ther 263 (1): 170-9 (1992)